��Ŀ����
����ij��ͭ��ͭп�Ͻ𣬼ٶ������ɷ־��������ᷴӦ����Ʒ�������ͼ��ѡ���ʵ���ʵ��װ�ã�������ʵ��ⶨ�û�ͭ��Ʒ��п�������������ش��������⣺
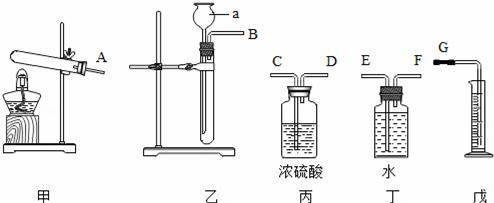

��1��д������a�����ƣ�������
��2��ʵ��Ҫѡ���װ������������װ�ñ�ţ����������������������������ӿڵ�˳��Ϊ������ӿ���ĸ���ţ���
��3��ϸ�Ĺ۲췢�֣�ʢװ��Һ���Լ�ƿ���ռ�����ļ���ƿ����ĥɰ���֣�����ͬ���Ǽ���ƿ��ĥɰ����λ��������
a��ƿ���ڱ� b��ƿ�ڱ�Ե c��ƿ���ڱ� d��ƿ�����
��4��������װ�ú��ڿ�ʼʵ��ʱ��Ҫ��������
��5����ʵ����Ʒ��������9.9g��ʵ���вⶨ�����������1.1L�������ܶ�Ϊ0.09g/L������û�ͭ��Ʒ��п����������Ϊ����
����������1�����ݳ������������ƺ���;���н��
��2������п�����ᷴӦ���ڹ����Һ���ϲ���Ҫ���ȵķ�Ӧ���н��
��3�����ݼ���ƿ���������н��
��4������������װ�ú��ڿ�ʼʵ��ʱ��Ҫ�ȼ��װ�õ������Խ��н��
��5�����������������������������������������п���������Ӷ�����û�ͭ��Ʒ��п�������������ɣ�
����𡿽⣺��1������a�����ƣ�����©����
�ʴ�Ϊ������©����
��2��п�����ᷴӦ���ڹ����Һ���ϲ���Ҫ���ȵķ�Ӧ����ʵ��Ҫ������������������ʵ��Ҫѡ���װ�����ҡ������죬�ӿڵ�˳��ΪB��F��E��G��
�ʴ�Ϊ���ҡ������죻B��F��E��G��
��3��ʢװ��Һ���Լ�ƿ���ռ�����ļ���ƿ����ĥɰ���֣�����ͬ���Ǽ���ƿ��ĥɰ����λ��ƿ�ڱ�Ե��
�ʴ�Ϊ��b��
��4��������װ�ú��ڿ�ʼʵ��ʱ��Ҫ�ȼ��װ�õ������ԣ�
�ʴ�Ϊ�����װ�õ������ԣ�
��5������������=1.1L��0.09g/L=0.099g��
�����п������Ϊx��
Zn+2HCl�TZnCl2+H2��
65 2
x 0.099g
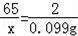

x=3.2175g
�û�ͭ��Ʒ��п����������=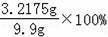
 =32.5%��
=32.5%��
�ʴ�Ϊ��32.5%��

 ��У����ϵ�д�
��У����ϵ�д�

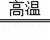
 2CO
2CO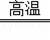
 ����Һ��һ������Al3+��Ag+ B����Һ��һ������Fe2+��Ag+
����Һ��һ������Al3+��Ag+ B����Һ��һ������Fe2+��Ag+