��Ŀ����
4���ձ���ʢ��һ���������¶�Ϊ80�桢����ΪM����Һ�������������»����У��ⶨ��ͬ�¶�ʱ��������M���������ⶨ�����¼���±���| ������Һ���¶�/�� | ��75 | ��65 | 50 | 35 | ����20 |
| ��������M������/g | ����0g | ��0g | 2.0g | 4.5g | 8.4g |
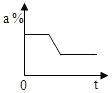
��1��75��ʱ������Һ�Ƿ�Ϊ������Һ�����DZ�����Һ
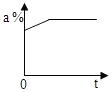
��2����40��ʱ�ӽ����͵�M��Һ��ɱ�����Һ�����з�����һ���ܴﵽĿ���Тۢݣ�����ţ���
�����¡��ڽ��¡��ۼ�����M �ܼ�ˮ���ݺ�������ˮ
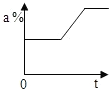
��3��20��ʱ���ù����ĩM��ˮ����100g������������Ϊ5%��M��Һ�������õ��������У�������ƽ��������У����ձ���ҩ�ס���Ͳ��100mL������ͷ�ιܲ�������
���� ����Ŀ����Ϣ��֪��75��ʱ������Һ�ǣ����DZ�����Һ����40��ʱ�ӽ����͵�M��Һ��ɱ�����Һ��һ���ܴﵽĿ���У�������M����������ˮ��20��ʱ���ù����ĩM��ˮ����100g������������Ϊ5%��M��Һ�������õ��������У�������ƽ��������У����ձ���ҩ�ס���Ͳ��100mL������ͷ�ιܣ���������
��� �⣺��1������Ŀ����Ϣ��֪��75��ʱ������Һ�ǣ����DZ�����Һ����Ϊ���º�û�й����������ʴ�Ϊ�����DZ�����Һ��
��2����40��ʱ�ӽ����͵�M��Һ��ɱ�����Һ��һ���ܴﵽĿ���У�������M����������ˮ���ʴ�Ϊ���ۢݣ�
��3��20��ʱ���ù����ĩM��ˮ����100g������������Ϊ5%��M��Һ�������õ��������У�������ƽ��������У����ձ���ҩ�ס���Ͳ��100mL������ͷ�ιܣ����������ʴ�Ϊ����������
���� ���⿼�����жϱ�����Һ�벻������Һת���ķ����������ܽ�����¶����߶���������ʣ��ɲ�������Һ��ɱ�����Һ�ķ����ȣ���������Ҫ������ѡ�����������У�

��ϰ��ϵ�д�
 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ
15���ճ������У���������̼������Һ���м��ԣ���ϴ�;��ϵ����ۣ�����Խǿ��ȥ���۵�Ч��Խ�ã������Ƕ�Ӱ��̼������Һ���Ե�����չ��̽����
��̼���ƹ���Ͳ�ͬ�¶ȵ�ˮ�������������������ֱ�Ϊ2%��6%��10%��̼������Һ������������Һ��pH����¼�������±���
��������������ݻش�
��1��ȥ���۵�Ч����õ��Ǣᣨ��ʵ���ţ���
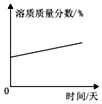
��2����һ���¶ȷ�Χ�ڣ��¶ȶ�̼������ҺpH��Ӱ���ǣ���̼������Һ������������ͬʱ���¶�Խ�ߣ�pHֵԽ��
��3��Ҫ����̼������Һ��pH����Һ���������������ı仯��ϵ���ߣ���ѡ���һ��ʵ����
�٢ܢߣ���ۢޢᣨ��ʵ���ţ�����������һ��������������Χ�ڣ��¶�һ��ʱ��������������Խ����ҺpHԽ��
��̼���ƹ���Ͳ�ͬ�¶ȵ�ˮ�������������������ֱ�Ϊ2%��6%��10%��̼������Һ������������Һ��pH����¼�������±���
| ʵ���� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
| ������������ | 2% | 2% | 2% | 6% | 6% | 6%] | 10% | 10% | 10% |
| ˮ���¶ȣ��棩 | 20 | 40 | 60 | 20 | 50 | 60 | 20 | 40 | 60 |
| ��ҺpH | 10.90 | 11.18 | 11.26 | 11.08 | 11.27 | 11.30 | 11.22 | 11.46 | 11.50 |
��1��ȥ���۵�Ч����õ��Ǣᣨ��ʵ���ţ���
��2����һ���¶ȷ�Χ�ڣ��¶ȶ�̼������ҺpH��Ӱ���ǣ���̼������Һ������������ͬʱ���¶�Խ�ߣ�pHֵԽ��
��3��Ҫ����̼������Һ��pH����Һ���������������ı仯��ϵ���ߣ���ѡ���һ��ʵ����
�٢ܢߣ���ۢޢᣨ��ʵ���ţ�����������һ��������������Χ�ڣ��¶�һ��ʱ��������������Խ����ҺpHԽ��
19������4��ͼ���У�����ȷ��Ӧ�仯��ϵ���ǣ�������
 |  |  |  |
| A����һƿŨ����¶���ڿ����� | B����һ��������ϡ�����м���пƬ | C������һ�������ĸ�����ع��� | D����һ���������Ȼ�þ�� ϡ����Ļ����Һ����μ�������������Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
9��һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�ϣ��ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��
��1��ú����ˮ������Ӧ��������CO��H2����Ӧ�Ļ�ѧ����ʽΪC+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��2����Ȼ�����������Եõ��ϳ��������۹���ʾ��ͼ������ʾ��
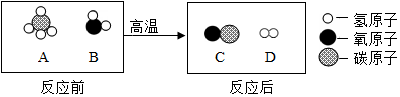
���������У�������C��D��������Ϊ14��3��
��3���ϳ�����CO��H2���ڲ�ͬ�����������£����Ժϳɲ�ͬ�����ʣ����Ժϳ���Ϊԭ�ϲ����ܵõ���������C������ĸ��ţ���
A�����ᣨH2C2O4�� B���״���CH3OH�� C������[CO��NH2��2]
��4�������±��ش�������⣮
��������������ʵ��ܽ����ȵ��¶ȷ�Χ����20��40�森
��20��ʱ������ص��ܽ����31.6g�����¶��£���20gKNO3����50gˮ�У���ֽ��裬������Һ��������65.8g��Ҫ��һ����߸���Һ�����������������ɽ��еIJ��������£�
��1��ú����ˮ������Ӧ��������CO��H2����Ӧ�Ļ�ѧ����ʽΪC+H2O$\frac{\underline{\;����\;}}{\;}$CO+H2��
��2����Ȼ�����������Եõ��ϳ��������۹���ʾ��ͼ������ʾ��

���������У�������C��D��������Ϊ14��3��
��3���ϳ�����CO��H2���ڲ�ͬ�����������£����Ժϳɲ�ͬ�����ʣ����Ժϳ���Ϊԭ�ϲ����ܵõ���������C������ĸ��ţ���
A�����ᣨH2C2O4�� B���״���CH3OH�� C������[CO��NH2��2]
��4�������±��ش�������⣮
| �¶ȣ��棩 | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�� ��g/100g ˮ�� | KNO3 | 13.3 | 31.6 | 63.9 | 110.0 | 169.0 | 246.0 |
| NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 | |
��20��ʱ������ص��ܽ����31.6g�����¶��£���20gKNO3����50gˮ�У���ֽ��裬������Һ��������65.8g��Ҫ��һ����߸���Һ�����������������ɽ��еIJ��������£�
16���±��г��˳�ȥ�����������������ʵķ��������в���ȷ���ǣ�������
| ���� | ���� | ��ȥ���ʵķ��� | |
| A | CaO | CaCO3 | ����������ˮ�ܽ⡢���� |
| B | NaOH��Һ | Na2CO3 | ����������Ca��OH��2��Һ������ |
| C | Cu��NO3��2��Һ | AgNO3 | ���������ͭ�ۣ����� |
| D | CO | CO2 | ͨ��NaOH��Һ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |