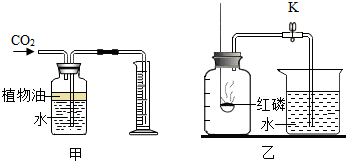
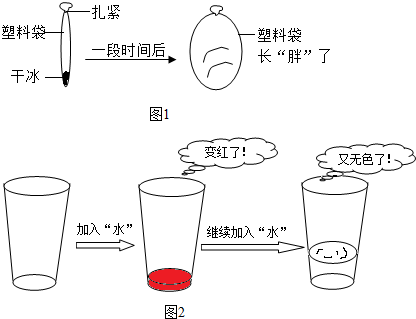
��Ŀ����
16��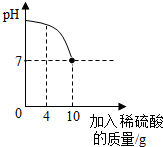 ʵ������һƿ��ǩ����ʴ��ϡ���ᣬΪ�˲ⶨ����Һ�����ʵ��������������ձ�������16g 10%������������Һ��Ȼ�����ձ��еμӸ�ϡ���ᣬ��Ӧ��������Һ��pH������ϡ����������ϵ��ͼ��ʾ����2NaOH+H2SO4�TNa2SO4+2H2O����������С�����2λ��
ʵ������һƿ��ǩ����ʴ��ϡ���ᣬΪ�˲ⶨ����Һ�����ʵ��������������ձ�������16g 10%������������Һ��Ȼ�����ձ��еμӸ�ϡ���ᣬ��Ӧ��������Һ��pH������ϡ����������ϵ��ͼ��ʾ����2NaOH+H2SO4�TNa2SO4+2H2O����������С�����2λ����1������16g 10%������������Һ����Ҫˮ������Ϊ14.4g��
��2����ϡ�������������������Ҫ���ݻ�ѧ����ʽ���㣬��д��������̣���
��3��������4gϡ����ʱ���ձ�����Һ����Ԫ�ص�������
���� ��1��������Һ����������=��Һ��������Һ�����ʵ���������������Ҫ������Һ����������������������������Ҫ�����������ܼ�������
��2�������������Ƶ�������ϻ�ѧ����ʽ�����������������������ϡ�������������������
��3�����������غ㶨�ɷ�����
��� �⣺��1����Ҫ�������ƹ��������16g��10%=1.6g����Ҫˮ������Ϊ16g-1.6g=14.4g��
��2������16g 10%������������Һǡ����ȫ��Ӧ�����������Ϊx
2NaOH+H2SO4=Na2SO4+2H2O
80 98
1.6g x
$\frac{80}{1.6g}$=$\frac{98}{x}$
x=1.96g
��ϡ�����������������$\frac{1.96g}{10g}$��100%=19.6%
�𣺸�ϡ�����������������19.6%
��3�����������غ㶨�ɣ���Ԫ�ص������ڷ�Ӧ�����в���
1.6g��$\frac{23}{40}$��100%=0.92g
����Ԫ�ص�����0.92g
�ʴ�Ϊ����1��14.4����2��19.6%����3��0.92g��
���� ������Ҫ����ѧ�����û�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ��

��ϰ��ϵ�д�
 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ
4���й�ʵ�����������������ǣ�������
| A�� | ���ڿ�����ȼ�շ�������ɫ���� | |
| B�� | þ�ڿ�����ȼ�գ�����ҫ�۰⣬�ų����������������İ��� | |
| C�� | ��ʢ��Ũ������Լ�ƿ�ĸ��Ӵ�ƿ���Ϸ�����ְ��� | |
| D�� | ������ͭ��Һ�еμ�����������Һ������ɫ���� |
5������ʳ���У����������ʵ��ǣ�������
| A�� | ���� | B�� | ���� | C�� | ���� | D�� | ���� |