��Ŀ����
16��ͼ����A��һ�ֳ������ᣬ�������ɫ����B��Ӧ��B����Ϊ8�ˣ���NaOH��Һ�������뵽C�У�������ɫ����E�����������NaOH��Һ��������ϵ��ͼ����ʾ����D�м���Ba��NO3��2��Һ������һ�ֲ�����ϡ����İ�ɫ������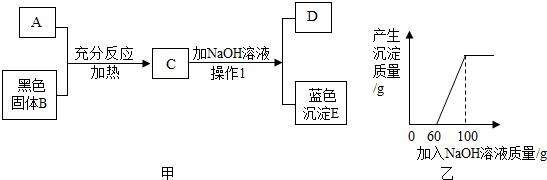
��1��A��Һ�����ʵĻ�ѧʽΪH2SO4��
��2������1�������ǹ��ˣ�
��3��NaOH��Һ�����ʵ����������Ƕ��٣�
��4��C��Һ�м���NaOH��Һ��ǡ����ȫ��Ӧʱ��������Һ���ʵ������Ƕ��٣�
���� ��1���������ʼ䷴Ӧ�Ĺ����Լ����ʵ���ɫ����ȷ�����ʵ����ƣ�
��2������1�Ƿ�����������Һ��������ͭ��ɫ������һ�ַ�����
��3����������ͭ���������������ͭ��������Ȼ���������ͭ�������������֮��Ӧ�����������Ƶ�����������ͼ���֪������ͭ��Ӧ������������Һ������Ϊ��100g-60g���������������������Һ�����ʵ�����������
��4����ͼ���֪��C��Һ�е�����Ϊ���������ͭ�������������������߾���Ӧ�����û�ѧ����ʽ�ֱ����������Ƶ��������ɣ�
��� �⣺��1����D�м���Ba��NO3��2��Һ������һ�ֲ�����ϡ����İ�ɫ����������D��Һ��һ��������������ӣ�E����ɫ����������������ͭ����ôC������ͭ��D�������ƣ���ɫ����Ϊ����ͭ����Ϊϡ���ᣬ���H2SO4��
��2������1�Ƿ��������Թ����Һ���һ�ַ������ǹ��ˣ�������ˣ�
��3���⣺����CuO���ɵ�CuSO4������Ϊx
CuO+H2SO4=CuSO4+H2O
80 160
8g x
$\frac{80}{160}=\frac{8g}{x}$
x=16g
����CuSO4��Ӧ��NaOH������Ϊy��ͬʱ����Na2SO4������Ϊa
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
160 80 142
16g y a
$\frac{160}{80}=\frac{16g}{y}$
y=8g
��������������Һ�����ʵ���������Ϊ��$\frac{8g}{100g-60g}��100%$=20%
$\frac{160}{142}=\frac{16g}{a}$
a=14.2g
��NaOH��Һ�����ʵ�����������20%��
��4���⣺���������������Ʒ�Ӧ��������������Ϊb
H2SO4+2NaOH=Na2SO4+2H2O
80 142
60g��20% b
$\frac{80}{142}=\frac{60g��20%}{b}$
b=21.3g
������Һ�����ʵ�����Ϊ��21.3g+14.2g=35.5g
��C��Һ�м���NaOH��Һ��ǡ����ȫ��Ӧʱ��������Һ���ʵ�������35.5g��
���� �����㿼���˸��ݻ�ѧ����ʽ�ļ�������������ļ��㣬�ǿ��Լ������о������ֵ����ͣ�����ʱҪע�⣺��ѧ����ʽҪд��ȷ��ʼ�ղ�Ҫ���������غ㶨�ɣ�

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д��ٽ�����Һ�ֱ�������������Һ��ϣ����۲쵽��ɫ�������ɣ�
�ڽ��ҡ�����Һ��ϣ������ݲ�����
�������Һ�е���AgNO3��Һ�������Թ۲쵽��ɫ��������ϡ�����������ʧ��
�������˰��õ������½��ۣ�����ȷ���ǣ�������
| A�� | ����Һ��һ������Ba2+ | B�� | ����Һ�п��ܺ���SO42- | ||
| C�� | ����Һ��һ������Cl- | D�� | ����Һ��һ������Na+ |
| A�� | �ڸ�������Ϳ������Է�ֹ������ | |
| B�� | 10mL�ƾ���10mLˮ��Ϻ����С��20mL������Ϊ���ӱ�С�� | |
| C�� | ����ɭ�ֻ���ʱ���ɿ��ڷ�����������Ŀ���Ǹ�����ȼ�� | |
| D�� | ��Ǧ��Ͻ���������˿������Ϊ�۵�� |
| ��Ӧǰ���������� | ��Ӧ����������� |
| 13.5g | 8.7g |
��2�����������Ʒ�е����������Ƕ��٣�
 ��ijԭ�ӵĽṹʾ��ͼ�����й��ڸ�ԭ�ӵ�������ȷ���ǣ�������
��ijԭ�ӵĽṹʾ��ͼ�����й��ڸ�ԭ�ӵ�������ȷ���ǣ�������| A�� | ���õ����� | B�� | ���ڷǽ���ԭ�� | ||
| C�� | �˵����Ϊ11 | D�� | ����������Ϊ11 |
| A�� | ��Ͳ�������кͷ�Ӧ������ | |
| B�� | �ձ�����ʱӦ������ʯ������ | |
| C�� | �¶ȼƿ����ڽ�����Һ | |
| D�� | Ϩ��ƾ��ƣ����õ�ñ����Ҳ�����촵�� |
| A�� | C+O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2 | B�� | Mg+2HCl�TMgCl2+H2�� | ||
| C�� | CaO+H2O�TCa��OH��2 | D�� | CO2+C$\frac{\underline{\;����\;}}{\;}$2CO |
 ���Ʋ�ϡ��һ������������Na2SO4��Һ��
���Ʋ�ϡ��һ������������Na2SO4��Һ��