��Ŀ����
18�� 2015��4��6��λ�ڸ������ݵ�PX��ĿͻȻ���ը�������˹�ע��PXѧ���Զ��ױ�������ɺͽṹ��ͼ��ʾ��PX����ʯ����Ʒ����Ҫ������ȡ����������ά��ԭ�Ͼ�����֬��
2015��4��6��λ�ڸ������ݵ�PX��ĿͻȻ���ը�������˹�ע��PXѧ���Զ��ױ�������ɺͽṹ��ͼ��ʾ��PX����ʯ����Ʒ����Ҫ������ȡ����������ά��ԭ�Ͼ�����֬����1��д��PX�ڿ�������ȫȼ�յĻ�ѧ����ʽ2C8H18+25O2$\frac{\underline{\;��ȼ\;}}{\;}$18H2O+16CO2��
�˴δ������������300�ֺ�CO2��ĭ��ȼ�յĴ���ʵʩ������������ԭ����ʹ��ȼ��������������
��2�����������ֵ�����ά�Ͳ�˿�ɲ��÷����ǣ�ȡ�������գ������ս���ëζ����Ϊ��˿����֮Ϊ������ά��
��3��������֬�����л��߷��ӻ�������в������л��߷��ӻ��������D��
A���ϳ��� B����ά�� C������ϩ D���ֲ�
��4�����������ϡ���ɽ��ⱻ�㷺���������ͨ���ϣ����������ϡ���ʹ�ÿɼ��ٰ�ɫ��Ⱦ��
���� ��1��������Ŀ����Ϣ��֪���͵��Ż��ϵ��Լ�������ȫȼ�ն�����̼��ˮ���н�𣻴��⿼������������ԭ�����������������к����Ľ��ͣ��ڽ��ʱ��Ӧ�����֪����ȼ�յ�����������ԭ����
��2�������շ������֣�
��3���л��߷��ӻ�������ָ��Է��������ܴ���л���ɴX������ʮ�������X��������
��4�����ⶪ��һ������ĭ���ϻ���ɰ�ɫ��Ⱦ��
��� �⣺��1������Ŀ����Ϣ������������һ���������ͷ���ʱ��һ��С���Ǿ�����ʹ��ȼ�տ�֪����˵�����͵��Ż��ϵͣ�������ȫȼ�ն�����̼��ˮ����Ӧ����ʽΪ��2C8H18+25O2$\frac{\underline{\;��ȼ\;}}{\;}$18H2O+16CO2��
����ȼ�յ��������ٿ�ȼ���������������������¶ȴﵽ�Ż�㣻�Լ���������ԭ������������ȼ��ڸ�������������������۽��µ��Ż�����£�ֻҪ����ȼ������������һ�����ɣ����ú�CO2��ĭ��ȼ�յĴ���ʵʩ��������ԭ���Ǹ���������
��2�����ֵ�����ά�Ͳ�˿�ɲ��÷���ȡ�������գ������ս���ëζ����Ϊ��˿����֮Ϊ������ά��
��3���ֲIJ������л��߷��ӻ�����ϳ�����ά�ء�����ϩ�������л��߷��ӻ����
��4�����ⶪ��һ������ĭ���ϻ���ɰ�ɫ��Ⱦ�����ԡ��������ϡ���ʹ�ÿɼ��ٰ�ɫ��Ⱦ��
�ʴ�Ϊ����1��2C8H18+25O2$\frac{\underline{\;��ȼ\;}}{\;}$18H2O+16CO2ʹ��ȼ������������
��2��ȡ�������գ������ս���ëζ����Ϊ��˿����֮Ϊ������ά��
��3��D ��4����ɫ
���� ��ѧ��Դ���������Ҳ������������������������������������صĻ�ѧ֪ʶҲ����Ҫ���п��ȵ�֮һ��

| A�� | ��ͬ�ַ��ӹ��ɵ�����һ���Ǵ�������Դ�����һ������ͬ�ַ��ӹ��ɵ� | |
| B�� | �кͷ�Ӧ�����κ�ˮ�����������κ�ˮ�ķ�Ӧһ�����кͷ�Ӧ | |
| C�� | ̼������ϡ���ᷴӦ�������壬������ϡ���ᷴӦ�������������һ����̼���� | |
| D�� | ���»����ʱ�ܶ�ȡ���������������ʿ�������ԭ��������һ����̼�������dz��õĻ�ԭ�� |
 ��ͼ��A��B��C���ֹ������ʵ��ܽ�����ߣ�����˵��������ǣ�������
��ͼ��A��B��C���ֹ������ʵ��ܽ�����ߣ�����˵��������ǣ�������| A�� | t1��ʱ���ܽ������������B | |
| B�� | ��t2��ʱ����150g A���ʵı�����Һ���µ�t1�棬�������������Ϊ25g | |
| C�� | t2��ʱA��B��C�������ʵĻ����Һ100�ˣ������������������ľ���һ����C | |
| D�� | ��ͬһ�ַ�������ʹA��B��C�������ʵ���Һ���о����������÷����������ᾧ |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | ��ºϽ�����Ƴɵ�·����˿����Ϊ�����۵�� | |
| B�� | �кͷ�Ӧһ�����κ�ˮ���ɣ����κ�ˮ���ɵķ�Ӧ��һ�����кͷ�Ӧ | |
| C�� | ��ij�����м���ϡ���ᣬ�������ݲ�����˵���ù���һ����̼���� | |
| D�� | ��������̼ͨ����ɫʯ����Һ�У���ɫʯ����Һ�����ɫ |
 ���л�ѧ�м��ֳ�������֮���ת����ϵ��ͼ��ʾ��ʵ��������������������֮���ܹ�������Ӧ����ͷ��ʾһ������������һ������ת������֪A��CΪ��ɫ��ĩ��A��D�ķ�Ӧ��������ʱ���ҹ��鼮�о��м��أ�F��G�ķ�Ӧ�Ǵ���ȻӦ��̫������ɹ��ķ�����A��B��G����һϵ�и��ӵķ�Ӧ����E��
���л�ѧ�м��ֳ�������֮���ת����ϵ��ͼ��ʾ��ʵ��������������������֮���ܹ�������Ӧ����ͷ��ʾһ������������һ������ת������֪A��CΪ��ɫ��ĩ��A��D�ķ�Ӧ��������ʱ���ҹ��鼮�о��м��أ�F��G�ķ�Ӧ�Ǵ���ȻӦ��̫������ɹ��ķ�����A��B��G����һϵ�и��ӵķ�Ӧ����E��
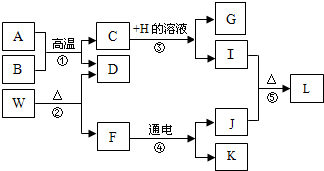 A��L�ֱ�Ϊ���꼶��ѧѧ���IJ�ͬ���ʣ����Ǵ�����ͼ��ʾ��ת����ϵ����֪AΪ�������Ҫ�ɷ֣�B��D��Ϊ��ɫ���壬E�����ֽ⣬F�ڳ�����ΪҺ�壬H����Һ����ɫ��
A��L�ֱ�Ϊ���꼶��ѧѧ���IJ�ͬ���ʣ����Ǵ�����ͼ��ʾ��ת����ϵ����֪AΪ�������Ҫ�ɷ֣�B��D��Ϊ��ɫ���壬E�����ֽ⣬F�ڳ�����ΪҺ�壬H����Һ����ɫ��