��Ŀ����
 һƿ���õ��������ƹ����Ѿ������˱��ʣ�ij�о���ѧϰС��Ϊ��̽�����ʳ̶ȣ��������²��룺���ܲ��ֱ��ʣ�������NaOH��Na2CO3�Ļ�������ȫ�����ʣ�������Na2CO3��
һƿ���õ��������ƹ����Ѿ������˱��ʣ�ij�о���ѧϰС��Ϊ��̽�����ʳ̶ȣ��������²��룺���ܲ��ֱ��ʣ�������NaOH��Na2CO3�Ļ�������ȫ�����ʣ�������Na2CO3����1�����ȶԹ���ijɷֽ���ȷ����ȡ�����������Թ��У���ˮ����ܽ⣬�ȼ���������BaCl2��Һ��������ɫ���������ú�ȡ�ϲ���Һ���ټ���CuSO4��Һ��������ɫ��״����������ʵ������ȷ���ù�����
��2����ȡ10.6g�ù�����Ʒ����ƿ�У�����һ������������ϡ���ᣬֱ���������õ��������±���
| ��Ʒ���� | ��Ӧǰ������ | ��Ӧ�������� |
| 10.6g | 148.5g | 146.3g |
��3����ȡ������Ʒ����ˮ������һ������������ϡ���ᣬֱ���������������ϡ��������������CO2����
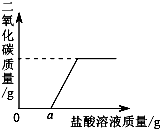
��������ϵ����ͼ��ʾ����ͼ�п����жϣ��ڸ���Ʒ��Һ�м���ϡ���ᣬ������֮��Ӧ��������
��4�����������һ��������NaOH���壬����ǰ������ͬ����������ϡ����ǡ����ȫ��Ӧ�������ǰ�����������
���㣺ҩƷ�Ƿ���ʵ�̽��,��Ļ�ѧ����,�εĻ�ѧ����,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�йػ�ѧ����ʽ�ļ���,��ѧ̽��
��������1������������BaCl2��Һ��������ɫ�������ð�ɫ������BaCO3��˵���ù���ɷ��к���CO32-���ټ���CuSO4��Һ��������ɫ��״����������ɫ��״������Cu��OH��2��˵���ù���ɷ��к���OH-��
��2�����������غ㶨�ɿ���֪����Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼���������������ɵĶ�����̼��������Ϸ�Ӧ�Ļ�ѧ����ʽ���������̼���Ƶ��������������̼���Ƶ�����������
��3��̼���������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ͼ��������ʾ����ʼ����ϡ����ʱ��û��������������жϴ�ʱ����û�к�̼���Ʒ�����Ӧ��
��4�����ݻ�ѧ�仯ǰ��Ԫ�ص��������䣬�������Ʊ�������̼����ʱ���ʵ��������ӣ�������������Ԫ�ص��������䣬���ַ�Ӧ�������Ȼ��Ƶ�������ȣ��ɵ�֪����ϡ���������Ҳ��ȣ�
��2�����������غ㶨�ɿ���֪����Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼���������������ɵĶ�����̼��������Ϸ�Ӧ�Ļ�ѧ����ʽ���������̼���Ƶ��������������̼���Ƶ�����������
��3��̼���������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ͼ��������ʾ����ʼ����ϡ����ʱ��û��������������жϴ�ʱ����û�к�̼���Ʒ�����Ӧ��
��4�����ݻ�ѧ�仯ǰ��Ԫ�ص��������䣬�������Ʊ�������̼����ʱ���ʵ��������ӣ�������������Ԫ�ص��������䣬���ַ�Ӧ�������Ȼ��Ƶ�������ȣ��ɵ�֪����ϡ���������Ҳ��ȣ�
����⣺��1����������֪���ù�����Һ�к���CO32-��OH-��������ƿ���õ��������ƹ�����NaOH��Na2CO3�Ļ���
���NaOH��Na2CO3�Ļ���
��2�����������غ㶨�ɿ���֪����Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼��������������Ϊ��148.5g-146.3g=2.2g��
����Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
=
��ã�x=5.3g
����Ʒ��Na2CO3����������Ϊ
��100%=50%
�𣺸���Ʒ��Na2CO3����������Ϊ50%��
��3�����ݼ���ϡ��������������CO2�����������ϵͼ��֪����һ��ʼ����ϡ����ʱ��û�����������˵����ʱ����ϡ�������������Ʒ�����Ӧ�����NaOH��
��4������ǰ����Ԫ���������䣬�����������ַ�Ӧ�����ɵ��Ȼ��Ƶ�����һ����ȣ�����ȵ��Ȼ����������ӵ�������ͬ�����ԣ�����ǰ��������ϡ�����������ȣ�������ڣ�
���NaOH��Na2CO3�Ļ���
��2�����������غ㶨�ɿ���֪����Ӧǰ����ٵ�����Ϊ���ɵĶ�����̼��������������Ϊ��148.5g-146.3g=2.2g��
����Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
| 106 |
| 44 |
| x |
| 2.2g |
��ã�x=5.3g
����Ʒ��Na2CO3����������Ϊ
| 5.3g |
| 10.6g |
�𣺸���Ʒ��Na2CO3����������Ϊ50%��
��3�����ݼ���ϡ��������������CO2�����������ϵͼ��֪����һ��ʼ����ϡ����ʱ��û�����������˵����ʱ����ϡ�������������Ʒ�����Ӧ�����NaOH��
��4������ǰ����Ԫ���������䣬�����������ַ�Ӧ�����ɵ��Ȼ��Ƶ�����һ����ȣ�����ȵ��Ȼ����������ӵ�������ͬ�����ԣ�����ǰ��������ϡ�����������ȣ�������ڣ�
������Ҫ���������ڣ�2�������Ŀ�����ȣ�Ҫ�������Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㲽���ʽ���Լ���֮��ص�֪ʶ�ȣ�Ȼ���������������龰�������ѧ�����֪ʶ�ͼ��ܣ�ϸ�µط������Ⲣϸ�ĵ�̽��������������ĿҪ����������ѡ����ɣ�

��ϰ��ϵ�д�
 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ
����Ƭ����������Һ�У�Ƭ�̺�ȡ������Һ�������ٵ��ǣ�������
| A������ | B������ͭ |
| C��ϡ���� | D���������� |
�ճ������е����б仯�����������ֱ仯���ű���������ǣ�������
| A���������� |
| B��Ũ����ϡ�� |
| C��������Һ���� |
| D���������Ƴ��� |
���и������ʼ䷴Ӧ��Ҫ�������ָʾ�������жϳ���Ӧ�������ǣ�������
| A��п��ϡ���� |
| B���ռ���ϡ���� |
| C��������ͭ��ϡ���� |
| D������ͭ��Һ������������Һ |
 С����֤������������˫��ˮ�ķֽⷴӦ��������ã��������ڷ�Ӧǰ�������Ƿ����仯��������ͼʵ�飮
С����֤������������˫��ˮ�ķֽⷴӦ��������ã��������ڷ�Ӧǰ�������Ƿ����仯��������ͼʵ�飮
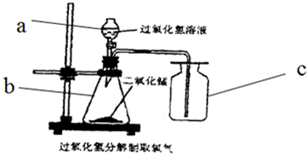 ʵ�����У������÷ֽ����������Һ����������������������ȸ�����صķ�����ȡ������
ʵ�����У������÷ֽ����������Һ����������������������ȸ�����صķ�����ȡ������