��Ŀ����
4��ľ̿��ԭ����ͭʵ���Ļ�Ϸ�ĩ�к���ͭ������ͭ��ľ̿�ۣ�ij��ѧС��ͬѧ��ƻ���ͭ�ķ������£�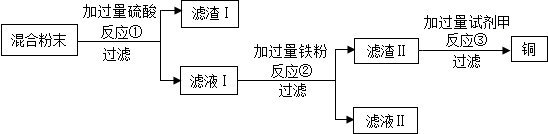
�ٹ��˲����б����õ��IJ��������У��ձ�����������©�������в�������������������
�ڷ�Ӧ�ٵĻ�ѧ����ʽΪH2SO4+CuO�TCuSO4+H2O��
�۷�Ӧ�������û���Ӧ��Ӧ����д������Ӧ���ͣ�����ҺII �е�����ΪFeSO4����д��ѧʽ����
������H2SO4��CuSO4��ѡ���Լ��ף����ѡ��H2SO4��������ԭ����C����д��ţ���
A�����Խ�����ͭ��Ϊͭ
B����Ȼ�������������ͭ�࣬�����
C��������ͭ��Ӧһ��ʱ�����������ͭ���谭�����ܽ�
D����������ͭ����Ϊ��������ͭ������ؽ�����Ⱦ
���Լ���ѡ�����ᣬʵ������У�����������������ð������д���������ж����������
���� ��1�����ݹ���ʱ���������������������ý��
��2�����ݷ�Ӧ���̼���Ӧ���������д����ѧ����ʽ��
��3�����ݷ�Ӧ���̷������
��4�����ݷ�Ӧ����Һ����Ⱦ�Է�����
��5��Ҫ�õ������ͭ���ɽ���Ӧ�����е�ͭȫ�����գ�
��� �⣺
��1�����ݹ���ʱ����������֪�������õ��IJ����������ձ�����������©�������в�����������������
��2������ͭ�����ᷴӦ��������ͭ��ˮ��ͭ��ľ̿�������Ӧ����ѧ����ʽΪ��H2SO4+CuO�TCuSO4+H2O��
��3����Ӧ�ٺ���Һ1�е����������������ͭ�����˺����Һ�м���������ۣ�����ͭ�����۷�Ӧ��������������ͭ����ѧ����ʽΪ��Fe+CuSO4�TFeSO4+Cu��H2SO4+Fe�TFeSO4+H2�������û���Ӧ����Һ���е�����Ϊ����������
��4���������к���ʣ������ۣ������ۺ�õ�ͭ����֪��ȥ���������ۣ������������Һ��������ͭ��Ӧһ��ʱ�����������ͭ���谭�����ܽ⣻
��5���������������ᷴӦ�����������ῴ��������ð����������������ð�������������
�ʴ�Ϊ��
��1��©����������
��2��H2SO4+CuO�TCuSO4+H2O��
��3���û���Ӧ��FeSO4��
��4��C��
��5��������������
���� ��������ͼ����Ŀ�ؽ��ǿ���������ͼ�и����ʵ�ת����ϵ��ע��������ʵ�ȥ����γ�ȥ�������ʣ�

| A�� | �������������������11�˻�33�� | |
| B�� | �����еĺ�ɫ���ʿ�����32�˻�96�� | |
| C�� | ���뷴Ӧ������ͭ����������40�˻�120�� | |
| D�� | ϡ�������������������38.4�˻�115.2�� |
| �¶ȣ��棩 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
| �ܽ�ȣ�g/100gˮ�� | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | 138 | 169 | 202 | 246 |

