��Ŀ����
�±�ΪijƷ������Ƭ��ǩ�е�һ���֡�
| Ӫ���ɷ� | ������(g) | ����(g) | ֬��(g) | ��(mg) | ��(mg) | ��(mg) | п(mg) | ά����C(mg) |
| ÿ100g�� | 7.4 | 7.8 | 7.9 | 206 | 19.2 | 37.8 | 10.1 | 18 |
(1)�ϱ�������������Ӫ�����е�  �ࡣ
�ࡣ
(2)������ȱ�������� ��
(3)���彡���벻����Ԫ�ء������йظ�Ԫ�ص������У�����ȷ����(�����) ��
A�������и�Ԫ�شִ����ڹ� ����������
����������
B������������ȱ�ƻ�����Ͳ��ͷ�������
C��������ȱ�ƻᷢ���������ɣ������ۡ���
D�������˱�������������Ҫ�������ĸ�
(4)����ÿ�˵�������ȫ�����ų�����ԼΪ18 kJ����ÿ100 g����Ƭ�е�������ȫ�������ų�������ԼΪ kJ��
��1���壬��2��ȱ����ƶѪ����3��D����4��3.6

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д���ѧУ��Ԫ���������ϣ�ijͬѧ�����ˡ�ˮ�����𡱵�ħ����������й������ƣ�Na2O2����ĩ����֬���ϵ�ˮ����֬��ȼ��������С���ܸ���Ȥ�����ǣ�����ͬѧ�ǽ���̽����
[�������]����������ˮ��Ӧ������ʲô���ʣ�
Ϊʲô��֬��ȼ�գ�
 [����]�ٿ�����һ���������һ����������
[����]�ٿ�����һ���������һ����������
�ڷ�Ӧ�����п����������仯
[���װ��]����ͼ��ʾ
[ʵ��̽��]
ʵ��һ��̽����Ӧ�����ɵ�������ʲô��
�Ŵ���ͼװ���з�Һ©���Ļ��������Ƶμ�ˮ���ٶȣ��۲쵽�Թ��������ݲ������ô����ǵ�ľ������P����ľ����ȼ��˵�����ɵ������� ��
��ʵ���У����۲쵽�����ձ��еĵ��ܿ�������ð����
����Ͳ����������ԭ��
ʵ���������̽����Ӧ�����ɵ���һ����ʲô��
��С�Ų�����һ��������Na2CO3��С����Ϊ�����ܡ�С���������ǣ�
Ϊ��֤ʵС���Ŀ������������һ��֤��CO32-��������ʵ�顣(2��)
| ʵ�鲽�� | ʵ������ | ʵ����� |
��С��ȡ��Ӧ�����õ���Һ���Թ��У�������ɫ��̪��Һ�����ַ�̪��Һ���ɫ��˵����Ӧ�����õ���Һ�� �ԡ�
[����]��ʵ��̽���Ľ����д���������ƺ�ˮ��Ӧ�Ļ�ѧ����ʽ
2012��1�£����������ӷ�������Ũ�ȳ����¼������ݸ��¼�ij��ѧ�С�鿪չ��һ�ι��ڽ�������ͭ�������˳���̽��������Ⱦ�¹ʴ������������ֻ���������ϣ����ӣ�Cd����һ������ɫ�������ڻ�������ͨ������Ϊ+2��
��ˮ������Ԫ��ͨ�����Ȼ��ӵ� ��ʽ���� ���Ȼ���������ˮ
��ʽ���� ���Ȼ���������ˮ
��һ����������ͭ�������˳���̽��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ�����ӡ�ͭ�������Թ��У���������ϡ���� | �ӱ��������ݲ�����ͭ�������������ݲ��� | ____________________ |
| ȡ����ͭ���Թ��У�����������������Һ | �� �� | ͭ�Ļ�Դ����� |
ʵ����ۣ��ӡ�ͭ�������˳��___________________________��
���ش����⡿
(1)ʵ��֮ǰ��Ҫ��ɰֽ��ĥ����Ƭ��Ŀ����____________________________��
(2)д��ͭ����������Ӧ�Ļ�ѧ����ʽ__________________________________��
(3)С����ΪֻҪѡ������ҩƷ����ʵ����ܵó��������ֽ����Ļ�Դ�С��������ҩƷ������________________________________________����2�֣�

 ��Ӧ�������(����)��
��Ӧ�������(����)�� �� ��
�� ��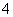 ��Ũ�ȸ�����ԭ����
��Ũ�ȸ�����ԭ����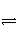
 Ag��(aq)��Cl��(aq)
Ag��(aq)��Cl��(aq)