��Ŀ����
Ϊ�ⶨ̼���ƺ��Ȼ��ƵĻ�������������ʵ������ȣ���������ʵ�飬ʵ�����̺����ݼ�¼��ͼ��ʾ���ش��������⣺

��1��д�����������з�����Ӧ�Ļ�ѧ����ʽ��__________________________________��
��2�����������غ㶨�ɣ��������ݿ�֪��������������������Ϊ__________��
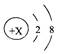
��3�����ݳ��������г��μӷ�Ӧ���Ȼ���������x���ı���ʽΪ__________________��
��4����Ʒ��̼�������Ȼ��Ƶ�������Ϊ_________________��
��5����Ӧ�����Һ�м���1.6gˮ��������Һ�����ʵ���������Ϊ___________��
��1��CaCl2+ Na2CO3=CaCO3��+ 2NaCl
��2��10g
��3��111/100=x/10g��100/111=10g/x
��4��106:117
��5��11.7%

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ
��������ʵ��ԭ�������ȷ���ǣ� ��
| ѡ�� | ��ʵ | ���� |
| A | ���ʯ�ʵؼ�Ӳ����ʯī���� | ̼ԭ�ӵĽṹ��ͬ |
| B | �ɱ����DZ� | �ɱ��ͱ��Dz�ͬ���ӹ��ɵ� |
| C | 10mL�ƾ���10mL�ƾ���Ϻ��������20mL | �ƾ����Ӽ�û�м�� |
| D | ����Һ����ʹ��ɫ��̪���ɫ | ����Һ�ж����н������� |
 ������һ������ԭ�ӣ���Ϊ̽��̼����淋����ȶ��ԣ��������м���ʵ�飻�ܽ����ܶ���Ҫ���ƻ����飬��Ϊ������̼���ж��ԣ���Ϊ�۲����þ����ɫ����ɰֽ��ĥþ������ƿ�ϵĵι�ʹ�ù�������ϴ��ֱ�ӷŻ�ԭƿ��������ȷ���ǣ� ��
������һ������ԭ�ӣ���Ϊ̽��̼����淋����ȶ��ԣ��������м���ʵ�飻�ܽ����ܶ���Ҫ���ƻ����飬��Ϊ������̼���ж��ԣ���Ϊ�۲����þ����ɫ����ɰֽ��ĥþ������ƿ�ϵĵι�ʹ�ù�������ϴ��ֱ�ӷŻ�ԭƿ��������ȷ���ǣ� �� ���͡�
���͡� ���ֱ��ʾ���ֲ�ͬԪ�ص�ԭ�ӣ����б�ʾ�������ǣ� ��
���ֱ��ʾ���ֲ�ͬԪ�ص�ԭ�ӣ����б�ʾ�������ǣ� ��
 ��ͼ�ǵ����������ڷŵ������·������Ϸ�Ӧ����ģ��ͼ����ش��������⣺
��ͼ�ǵ����������ڷŵ������·������Ϸ�Ӧ����ģ��ͼ����ش��������⣺ ��������
�������� ���Ӻ�С B������������
���Ӻ�С B������������ ������KClO3��O2�������У��ټ��ȣ��ڼ��װ�������ԣ������Թ���װ��ҩƷ���ܵ�����������ʱ����ˮ���ռ�����ʵ����Ͻ������ܴ�ˮ����ȡ������Ϩ��ƾ��ƣ��߽��Թ̶ܹ�������̨�ϣ���ȷ˳����
������KClO3��O2�������У��ټ��ȣ��ڼ��װ�������ԣ������Թ���װ��ҩƷ���ܵ�����������ʱ����ˮ���ռ�����ʵ����Ͻ������ܴ�ˮ����ȡ������Ϩ��ƾ��ƣ��߽��Թ̶ܹ�������̨�ϣ���ȷ˳���� H4
H4