��Ŀ����
�������ƾ��壨CaO2•8H2O�����ȶ����ʰ�ɫ������ˮ���㷺Ӧ���ڻ���ɱ�����������Ա���Ϊԭ���Ʊ�CaO2�������£�

��1������X��CO2���������������������������������ƾ�������Һ����ķ�������������������
��2����ӦY������¶���0��5�棬�ɽ���Ӧ���������������������У��÷�Ӧ�ǻ��Ϸ�Ӧ����Ӧ������CaO2•8H2O����д����ѧ����ʽ����������������õĹ������ƾ����г�����Ca��OH��2���ʣ�ԭ������������������
��3��CaO2����Է�������Ϊ���������������������ƾ��壨CaO2•8H2O����H��OԪ�ص�������Ϊ��������������
��4��Ϊ�ⶨ�ƵõĹ������ƾ�����CaO2•8H2O��������������Ƶ�ʵ�����£���ȡ������Ʒ50g�����ȵ�220���ַ�Ӧ������ʽΪ2CaO2•8H2O 2CaO+O2��+16H2O�������ʲ������仯���������������������Ϊ3.2g���������Ʒ��CaO2•8H2O������������CaO2•8H2O��Է�������Ϊ216����д����Ҫ�ļ�����̣�
2CaO+O2��+16H2O�������ʲ������仯���������������������Ϊ3.2g���������Ʒ��CaO2•8H2O������������CaO2•8H2O��Է�������Ϊ216����д����Ҫ�ļ�����̣�
���㣺
���ʵ��ת�����Ʊ�����Է��������ĸ������㣻Ԫ�������ȵļ��㣻��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����ݻ�ѧ��Ӧ����ʽ�ļ��㣮
ר�⣺
���ʵ��Ʊ���
������
��1�����ݻ�����������Լ����������Һ��ķ�����������
��2�����ݷ�Ӧ��Ҫ���¶��Լ���Ӧ�Ĺ�����������
��3��������Է���������Ԫ�������ȵļ��㷽����������
��4���������������������û�ѧ����ʽ���м��㣮
���
�⣺��1�����������������������֪��CO2����������̼�����������Һ����ù��˵ķ��������������̼�����ˣ�
��2�������¶���0��5�棬�����ڱ�ˮ������н��У��÷�Ӧ�ķ�Ӧ���������ơ�ˮ�������⣬��������CaO2•8H2O����Ϊ����������ˮ��Ӧ�����������ƣ���������������ˮ�������ˮ����CaO+H2O2+7H2O=CaO2•8H2O��CaO��Ca��OH��2��������Ca��OH��2�ܣ�
��3��CaO2����Է�������Ϊ40+16��2=72���������ƾ��壨CaO2•8H2O����H��OԪ�ص�������Ϊ��1��16������16��10��=1��10�����72��1��10��
��4���⣺����Ʒ��CaO2•8H2O������Ϊx
2CaO2•8H2O 2CaO+O2��+16H2O��
2CaO+O2��+16H2O��
432 32
x 3.2g

x=43.2g
����Ʒ��CaO2•8H2O����������Ϊ =86.4%
=86.4%
����Ʒ��CaO2•8H2O����������Ϊ86.4%��
������
����ע�⿼�黯ѧ����ʽ����д��Ʒ���ȵ����㣬��ɴ��⣬������������ṩ����Ϣ���У���д����ʽҪע����ƽ��
��
��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д����и������ʱ仯�У�ÿһת����һ���������¾���һ��ʵ�ֵ��ǣ�������
| X | Y | Z | |
| A | Fe | FeCl2 | Fe2O3 |
| B | CO2 | Na2CO3 | CaCO3 |
| C | H2 | H2O | H2O2 |
| D | Ca��OH��2 | NaOH | NaCl |

| �� | A�� | A | B�� | B | C�� | C | D�� | D |
��
��嫵Ĵ����̲��ŷḻ����Դ��������ˮ��Դǰ��������̽��ѧϰС���Ժ���Ca2+��Mg2+��Cl﹣���ӵ�±ˮ�ͱ��ǣ���Ҫ�ɷ�ΪCaCO3��Ϊ��Ҫԭ����ʵ�����Ʊ���ˮCaCl2��������ͼ1��
��ش��������⣺

��1�����������õ��IJ����������ձ�������������������������
��2����MgCl2Ϊ��д��±ˮ�г�ȥMg2+ʱ������Ӧ�Ļ�ѧ����ʽ��������������
��3���������ữʱӦѡ�����������������������д��ѧʽ����
��4��������Ӧ��δ�漰�Ļ�����Ӧ����������������������д��ĸ��ţ�
a�����ֽⷴӦ b���û���Ӧ c�����Ϸ�Ӧ d���ֽⷴӦ
��5�����յ�CO2������������ʹ�������������ˮ������Ӧ������CH4��O2���÷�Ӧ�Ļ�ѧ����ʽΪ������������������������ʱ���ڲ�ͬ�������١��ڡ��ۣ��������£�CH4���������ʱ��ı仯��ͼ2��ʾ���ڵ�10СʱʱCH4�������������������������д���١������ڡ����ۡ�����
��6����֪T��ʱ���ֻ�������ˮ�к�Һ���е��ܽ�����±���
| AgNO3 | Ba��NO3��2 | AgCl | BaCl2 | |
| H2O��1�� | 170g | 92.0g | 1.50��10﹣4g | 33.3g |
| NH3��1�� | 86.0g | 97.2g | 0.80g | 0.00g |
����������������Һ���з������ֽⷴӦ�Ļ�ѧ����ʽΪ��������������
��
��ҵ�ϣ�ͨ������ת�����Ƶ�KClO3���壺NaCl��Һ NaClO3��Һ
NaClO3��Һ KClO3����
KClO3����
��1����ɢ��з�Ӧ�Ļ�ѧ����ʽ��NaCl+3H2O NaClO3+3��������������
NaClO3+3��������������
��2����������������ĸҺ��KClO3��������������������͡������͡�����Һ��д��ĸҺ�е��������ʵĻ�ѧʽ������������
��3������ͼװ�ã��г֡�����װ�����ԣ�����ʵ�飬��������������������Ϊ�жϢ��з����˻�ѧ��Ӧ�����ݵ���������������
| ѡ�� | ����ʵ�� | �������� |
| A | �����Ȼ��غͶ������̵Ļ���� | �����Թ��ڵĴ�����ľ����ȼ |
| B | ���ȼ�ʽ̼��ͭ���� | ����ʯ��ˮ����� |
| C | ����ͭ˿ | ����ˮ�е��ܿ�������ð�� |
| D | �����Ȼ�狀���ʯ�ҵĻ���� | ��̪��Һ��� |


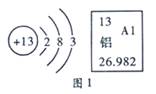 A. ���ڽ���Ԫ��
A. ���ڽ���Ԫ�� ȥ�����γ�Al3+
ȥ�����γ�Al3+