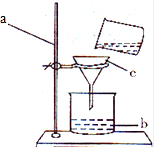
��Ŀ����
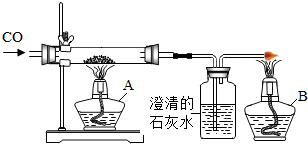
��ͼ����CO��ԭFe2O3 ʱ��ʵ�鿪ʼҪ���ڲ�������ͨ ���Ȳ������г���CO�ž��������� ��������ܻᷢ��װ�õ� ����ʵ�����ʱ��Ҫ��ֹͣ ���Ȳ����������ɵ�����ȴ�����ֹͣ ���������ȵ����ᱻ ��β������ֱ�����������ԭ���� ��ͼ�д���β���ķ����� ���������з�Ӧǰ�������仯���� ɫ��� ���������з�����Ӧ�Ļ�ѧ����ʽΪ ��
������������ΪFexOy���Ң�װ�����������Ӳ�ʲ����ܵ�����Ϊ20g����Ӳ�ʲ������������������������Ϊ23.6g���۹��ƿ�����ʯ��ˮ����������686g�� ��ͨ��CO������������������ȫ��ת��Ϊ�����ٳ�ʢʯ��ˮ�Ĺ��ƿ��������Ϊ688.2g����
�ٲ���C02������Ϊ ��
����������������Ԫ�غ���Ԫ�ص������� ����ѧʽΪ ��

������������ΪFexOy���Ң�װ�����������Ӳ�ʲ����ܵ�����Ϊ20g����Ӳ�ʲ������������������������Ϊ23.6g���۹��ƿ�����ʯ��ˮ����������686g�� ��ͨ��CO������������������ȫ��ת��Ϊ�����ٳ�ʢʯ��ˮ�Ĺ��ƿ��������Ϊ688.2g����
�ٲ���C02������Ϊ
����������������Ԫ�غ���Ԫ�ص�������
���㣺һ����̼��ԭ������,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺�������������
������һ����̼���������ڸ��������·�Ӧ�������Ͷ�����̼��
һ����̼���п�ȼ�ԣ��������������ϴﵽһ���̶�ʱ��������ᷢ����ը��
һ����̼�ж�����ɢ�������л���Ⱦ������
һ����̼ȼ�����ɶ�����̼��
��Ӧǰ����ƿ�������Ϊ��Ӧ���ɶ�����̼�����������ݶ�����̼�����������������������ȷ������������Ļ�ѧʽ������Ԫ�غ���Ԫ�ص������ȣ�
һ����̼���п�ȼ�ԣ��������������ϴﵽһ���̶�ʱ��������ᷢ����ը��
һ����̼�ж�����ɢ�������л���Ⱦ������
һ����̼ȼ�����ɶ�����̼��
��Ӧǰ����ƿ�������Ϊ��Ӧ���ɶ�����̼�����������ݶ�����̼�����������������������ȷ������������Ļ�ѧʽ������Ԫ�غ���Ԫ�ص������ȣ�
����⣺ʵ�鿪ʼҪ���ڲ�������ͨһ����̼���Ȳ������г���CO�ž��������ܼ��ȣ�������ܻᷢ��װ�õı�ը��
��ʵ�����ʱ��Ҫ��ֹͣ���ȣ��Ȳ����������ɵ�����ȴ�����ֹͣͨһ����̼���������ȵ����ᱻ�����е�����������
β������ֱ�����������ԭ����һ����̼�ж����ܹ���Ⱦ������
ͼ�д���β���ķ����ǵ�ȼ��
�������з�Ӧǰ�������仯���ɺ�ɫ��ɺ�ɫ��
�������з�����Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+3CO
2Fe+3CO2��
���һ����̼�����ȣ���ը�����ȣ�ͨһ����̼�������е�����������һ����̼�ж����ܹ���Ⱦ��������ȼ���죻��ɫ��Fe2O3+3CO
2Fe+3CO2��
�����������Ϊ��23.6g-20g=3.6g�����ɶ�����̼������Ϊ��688.2g-686g=2.2g��
FexOy+yCO
xFe+yCO2��
56x+16y 44y
3.6g 2.2g
=
��
=
��
��������������Ԫ�غ���Ԫ�ص�������Ϊ��
=56��16��
����������Ļ�ѧʽΪFeO��
���2.2g��56��16��FeO��
��ʵ�����ʱ��Ҫ��ֹͣ���ȣ��Ȳ����������ɵ�����ȴ�����ֹͣͨһ����̼���������ȵ����ᱻ�����е�����������
β������ֱ�����������ԭ����һ����̼�ж����ܹ���Ⱦ������
ͼ�д���β���ķ����ǵ�ȼ��
�������з�Ӧǰ�������仯���ɺ�ɫ��ɺ�ɫ��
�������з�����Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+3CO
| ||
���һ����̼�����ȣ���ը�����ȣ�ͨһ����̼�������е�����������һ����̼�ж����ܹ���Ⱦ��������ȼ���죻��ɫ��Fe2O3+3CO
| ||
�����������Ϊ��23.6g-20g=3.6g�����ɶ�����̼������Ϊ��688.2g-686g=2.2g��
FexOy+yCO
| ||
56x+16y 44y
3.6g 2.2g
| 56x+16y |
| 3.6g |
| 44y |
| 2.2g |
| x |
| y |
| 1 |
| 1 |
��������������Ԫ�غ���Ԫ�ص�������Ϊ��
| 56x |
| 16y |
����������Ļ�ѧʽΪFeO��
���2.2g��56��16��FeO��
�������������ʵ�飬��ѧ�ؽ���ʵ�顢����ʵ�飬�ǵó���ȷʵ����۵�ǰ�ᣬ���Ҫѧ�����ʵ�顢����ʵ�顢����ʵ�飬Ϊѧ�û�ѧ֪ʶ�춨������

��ϰ��ϵ�д�
�����Ŀ
����һЩ��ѧ��ʶ�����ǵ�����ϢϢ��أ���������������ǣ�������
| A�������������������ÿ��Ҫ����ʳ��5g���� |
| B��ҽ��������ˮ��0.5%���Ȼ�����Һ |
| C���������еĶ�����̼����������ﵽ1%ʱ����������к� |
| D��ͨ����ʳ����Լ��3%��5%�Ĵ��� |
���и������ʵĻ����ֱ���뵽ˮ�У����γ���ɫ����Һ���ǣ�������
| A��CaCl2��Na2CO3 |
| B��Na2SO4��Ba��OH��2 |
| C��FeCl2��Na2SO4 |
| D��BaCl2��NaOH |
���н��������ᷴӦ�������ݵ��ǣ�������
| A��ͭ | B���� | C���� | D���� |