��Ŀ����
9�� �Ƽ��仯��������ѧ��ѧѧϰ���о�����Ҫ���ݣ�
�Ƽ��仯��������ѧ��ѧѧϰ���о�����Ҫ���ݣ���1��ͼ1���Ƶ�ԭ�ӽṹʾ��ͼ������˵������ȷ����d
a�������ڽ���Ԫ�� b����ԭ�ӵ�������Ϊ11
c�������ӵķ���ΪNa+ d�����ڻ�ѧ��Ӧ���õ�����
��2�����ǻ��ý�����Ϊʲôͨ������ȴ����ʴ��
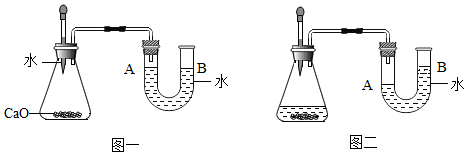
��3������һƿͼ2��Һ���Ȼ���4.5g������100g6%���Ȼ�����Һ����Ҫ�Ȼ��Ƶ�������6g��������Ͳ��ȡˮʱ���Ӷ���������������ȷ��������Һ������������6%�����������������=������
��4�����ᣨCH3COOH����һ�ֳ��õ��ᣬ�������ͨ�ԣ�����������������Һ��Ӧ���ɴ����ƺ�ˮ����ѧ����ʽΪ��CH3COOH+NaOH=CH3COONa+H2O•д���÷�Ӧ��ʵ�ʣ�
��5 ����6.5g��п��������ϡ���ᷴӦ���õ������������Ƕ��٣�
���� ��1��������ԭ�ӵĽṹͼ���з�����
��2�������ڿ������ױ��������ǣ�
��3��������������=��Һ���������������������з��������ݸ��Ӷ�����ʹ���������ݴ���ʵ�����ݽ��з�����
��4���ɴ���������������Һ��Ӧ���ɴ����ƺ�ˮ�Ļ�ѧ����ʽΪ��CH3COOH+NaOH�TCH3COONa+H2O��֪���÷�Ӧ����ʵ�������Ӻ����������ӽ��������ˮ��
��5������п��ϡ���ᷴӦ�Ļ�ѧ����ʽ������ʵ�������ϵ�������㼴�ɣ�
��� �⣺��1��a��ͨ��������֪����ԭ�Ӻ����11�����ӣ���A��ȷ��
b��ͨ��������֪�������ڵ�������Ԫ�أ���B��ȷ��
c����Ԫ�ص��������1�����ӣ�����ʧȥ���ӣ��γ�������Na+����C��ȷ��
d����Ԫ�ص��������1�����ӣ�����ʧȥ���ӣ���D����
��ѡ��d��
��2�����ڿ���������������Ӧ�������ܵ�����Ĥ�����������ı��棬��ֹ�˷�Ӧ�Ľ�һ�����У�
�ʴ�Ϊ�����ڿ���������������Ӧ�������ܵ�����Ĥ�����������ı��棬��ֹ�˷�Ӧ�Ľ�һ�����У�
��3���Ȼ��Ƶ�����Ϊ��500mL��1g/mL��0.9%=4.5g����100g6%���Ȼ�����Һ����Ҫ�Ȼ��Ƶ������ǣ�100mL��1g/mL��6%=6g�����Ӷ�����ʹ����������С��ʵ�����ݣ������ȡ��ˮ�࣬������Һ������������6%��
�ʴ�Ϊ��4.5��6������
��4���ɴ���������������Һ��Ӧ���ɴ����ƺ�ˮ�Ļ�ѧ����ʽΪ��CH3COOH+NaOH�TCH3COONa+H2O��֪���÷�Ӧ����ʵ�������Ӻ����������ӽ��������ˮ��
�ʴ�Ϊ�������Ӻ����������ӽ��������ˮ��
��5���⣺����������������Ϊx
Zn+H2SO4�TZnSO4+H2��
65 2
6.5g x
$\frac{65}{2}=\frac{6.5g}{x}$
x=0.2g
�𣺿��Ƶ�������������0.2g��
���� �����漰֪ʶ��϶࣬���ѶȲ����������е�֪ʶ���

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�| A�� | 4Fe+3O2$\stackrel{��ȼ}{��}$2Fe2O3 | B�� | S+O2$\stackrel{��ȼ}{��}$SO2 | ||
| C�� | H2+O2$\stackrel{��ȼ}{��}$H2O | D�� | C+O2$\stackrel{��ȼ}{��}$CO2�� |
| A�� |  | B�� |  | C�� |  | D�� |  |

| A�� | X��ʾN2 | |
| B�� | ��ԭ�ӵĽṹʾ��ͼ��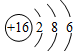 | |
| C�� | �����������С��������ΪC��X��O | |
| D�� | ̼ԭ�ӵ���������̼ԭ�����ӵ��������Ϻ�����ӵ����� |
| A�� | ���ͻӷ� | B�� | ��ҵ����ȡ���� | C�� | ��ʳ��� | D�� | ʯ���ۻ� |