��Ŀ����
����Ŀ��ʵ������Ũ���ᣨ������������Ϊ37%���ܶ�Ϊ1.18g.mL-1������120��������������Ϊ10%��ϡ���ᡣ
��1������:��ҪŨ����_____mL, ��д��������̣�����һλС������ͬ����
��2����ȡ:�ù��Ϊ_____mL����Ͳ����50mL��100 mL��ѡ����ȡŨ���ᡣ
��3�������������������800g10%ϡ���ᷴӦ����ų���������Ϊ���ٿ�_____������һλС����?
��4��С����ȡһƿ�������ľ��õ�������800g10%��ϡ���ᷴӦ��������ų�������������ԶԶС��������3������������������������ܵ�ԭ��_____��
���𰸡�����ҪŨ�������Ϊx������ϡ��ǰ�����ʵ��������䣬
x��1.18g/ mL��37%��120g��10%
x=27.5 mL 50 ��������������Ϊx

![]()
x=2.2g�� ���õ����ۿ����������⣨�������ɣ�
��������
��1������ҪŨ�������Ϊx������ϡ��ǰ�����ʵ��������䣬
x��1.18g/ mL��37%��120g��10%
x=27.5 mL
��2����ͲҪѡ��������̵ģ���ȡ27.5������Ӧѡ50������Ͳ��
��3����������������Ϊx

![]()
x=2.2g��
��4�����õ����ۿ����������أ��������ɣ��������������٣��������������١�

����Ŀ������þ������ˮ��Ӧ����������þ��������������Һ�ʼ��ԣ���ʹ��̪��Һ�Ժ�ɫ��ijͬѧ��þ������ˮ����У�����ȡ��������Һ���μӷ�̪��Һ����Ϊ��ɫ�������ڿ�����һ��ʱ�������Һ�ĺ�ɫ��ȥ�ˡ�
��1��þ����ˮ��Ӧ�Ļ�ѧ����ʽ��_____��
��2����������:��Һ��ɫ��ȥ��ԭ������Һ���Լ�����
[�������]������Һ���Լ�����ԭ����ʲô?
[���������]
����1:������������þ��N2��O2�����˷�Ӧ��
����2:��������Һ�����˿����е�_____��
����3:������������þ���ܽ�����¶ȵĽ��Ͷ�_____��
�ɼ�����ʿ�֪������1��������
[ʵ��̽��]
ʵ����� | ʵ������ | ���� |
1.ȡ������ɫ��Һ�������¶Ȳ��䡣�ڿ����з���һ��ʱ�䣬�۲����� | _____�� | ����2���� |
2.ȡ������ɫ��Һ��_____�۲����� | ��ɫ��ȥ | ����3���� |
[��չ����]
��3����һС�������Ͷ������ͭ��Һʱ��������ɫ�������������ԭ��:_____��
��4�������������⣬���ݽ������˳��Ԥ��_____Ҳ�ܸ�����ͭ��Һ��Ӧ������������һ�����ɣ���
����Ŀ��Ԫ����������ѧϰ���о���ѧ������Ϣ�ģ���ش�
��һ���� | 1H�� | 2He �� | |||||||
�ڶ����� | 3Li � | 4Be �� | 5B �� | 6C ̼ | 7N �� | 8O �� | 9F �� | 10Ne �� | |
�������� | 11Na �� | 12Mg þ | 13Al �� | 14Si �� | 15P �� | 16S �� | 17Cl �� | 18Ar � | |
��1����ԭ�ӽṹʾ��ͼΪ___________��
��2��̼Ԫ�غ���Ԫ����ʵ�������__________��
A��������ͬ
B��������ͬ
C������������ͬ
��3�� ��ʾ��ԭ���ڻ�ѧ��Ӧ����______���ʧ���á������ӣ���ԭ���γɵ����ӷ�����_______��
��ʾ��ԭ���ڻ�ѧ��Ӧ����______���ʧ���á������ӣ���ԭ���γɵ����ӷ�����_______��
��4���ڵ��������У�Ԫ�����͵ı仯����ǣ���������______Ԫ�أ��������ǽ�������ͬ�����ɵ�_____Ԫ�أ�����ϡ������Ԫ�ؽ�β��
��5��������ͬԭ�����͵������ķ��ӻ����ӽеȵ����塣���и��������ڵȵ�������� _______������ţ���
ANO��O2 BCO��N2 CSO2��CO2 DNH4+��H2O
����Ŀ������������۲졢ѧϰ����һ��ʵ�顣ȡ�Ķ���ʯ����ҺȾ����ɫ�ĸ����ֽ�����ֱ���ͼ����ʵ�顣
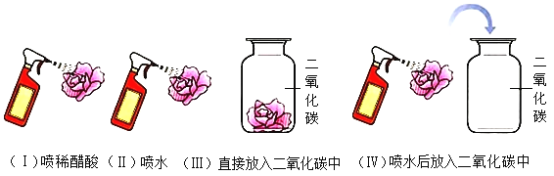
��1�������ͼʾ����˼�����ش��±��е����⣺
��I�� | ������ | ������ | ������ | |
���� | ��ɫֽ����� | ��ɫֽ������ɫ | ��ɫֽ������ɫ | _____�� |
���� | ��ʵ�飨������֤��_____����ʵ�飨������֤��_____�� |
��2��ʵ�飨����������ֽ���þƾ���С�ļ��ȹ�����ֽ���ֱ��ϣ�˵��̼��_____��д���÷�Ӧ�Ļ�ѧ����ʽΪ_____��



