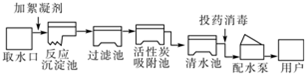
��Ŀ����
ˮ������֮Դ��������������ϵ���У�����ˮ�������䰮ˮ��Դ����ÿ������Ӧ�������κ�����
��1��Ϊ�˷�ֹˮ����Ⱦ���������������ڱ���ˮ��Դ����______�����ţ���������ˮ�����ж���ֲ����������ڲ������ŷŹ�ҵ��ˮ���۴���ʹ�û���ũҩ����������ˮ�����������������ŷţ�
��Ȼˮ�к����������ʣ�����ˮ���������У����ù��˵ķ�����ȥˮ�����������ʣ�ͬʱ���������������ClO2��һ�����͵�����ˮ����������ҵ����Cl2��NaClO2��ȡClO2������ƽ���л�ѧ����ʽ��______Cl2+______NaClO2�T2NaCl+______ClO2
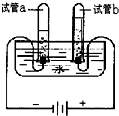
��2���ڻ�ѧʵ��Ϳ�ѧ�о��У�ˮҲ��һ�ֳ��õ��Լ���ˮ�������ض����������õ�һ��H+���γ�ˮ�������ӣ�H3O+�������ж��������̵���������������______������ţ���
A����Ԫ�ط����˸ı䡡��������������B��������ɷ����˸ı�
C�����Ļ�ѧ���ʷ����˸ı䡡������D����ˮ���ӱ�ˮ�����ӵĵ����������˸ı�
�о���Ա������֣���һ����ʵ�������£���ˮʩ��һ�����糡���ڳ��¡���ѹ�£�ˮ���Խ�ɱ�����Ϊ���ȱ��������ȱ���������ʵ�ü�ֵ���翪����ҩ���������״�ӡ���ȣ�����������______����д��ţ�
A������ɱ�����B����ֹ�����ۻ�����C������������������ D�������������
��3��ҽҩ�Ͽ���ʯ�����̶����۲�λ��ʯ������ʯ�ࣨCaSO4? H2O��һ�ְ�ɫ��ĩ������ʯ�ࣨCaSO4?2H2O��һ�ּ�Ӳ�Ĺ��壩���֣��̶����۵�ʯ����______���ѧʽ�����̶�ʱ������Ӧ�Ļ�ѧ����ʽ______��
H2O��һ�ְ�ɫ��ĩ������ʯ�ࣨCaSO4?2H2O��һ�ּ�Ӳ�Ĺ��壩���֣��̶����۵�ʯ����______���ѧʽ�����̶�ʱ������Ӧ�Ļ�ѧ����ʽ______��
�⣺��1������ˮ��Դ�Ĵ�ʩ�����ᵼ��ˮ��Ⱦ������������ʹ�û���ũҩ�����ڱ���ˮ��Դ������ˮ�����ж���ֲ���������ʹˮ�ʱ仵���Ӷ�ʹˮ����Ⱦ����ѡ�ڢܣ����ݻ�ѧ��Ӧǰ��ԭ�ӵ���������������ԭ�����ù۲취���ԶԻ�ѧ����ʽ������ƽ����ϵ��Ӧ�ֱ�Ϊ��1��2��2��
��2��H+��ˮ����γ�ˮ�������ӵķ���ʽΪ��H++H2O=H3O+�����������غ㶨�ɿ�֪��Ԫ������û�б仯��Ԫ�ػ��ϼ�Ҳû�б仯��������ɷ��Ӻ����ӽ�ϳ�Ϊ���Ӷ������仯����ѧ����Ҳ��Ϊ���ԣ�������ǰ�����������Ϊ10���ӣ��ޱ仯����ѡ��A��D��
�����ȱ��ĸ�����γ�������֪���ȱ�ʵ����Ȼ��ˮ���ʲ���������ɱ��ͷ�ֹ�����ۻ�����;�������ȱ����ص㡰�ڳ��¡���ѹ�£�ˮ���Խ�ɱ�����֪�������¿������������ܽ�������������ʴ�Ϊ��C��D��
��3��ҽҩ�Ͽ�����ʯ�����̶����۲�λ����ʯ����һ�����¶��º�ˮ��ϱ�Ϊ�ϴ�Ӳ�ȵ���ʯ�࣬�Ӷ��̶����ã�����ʽΪ��2��CaSO4? H2O��+3H2O=2��CaSO4?2H2O����
H2O��+3H2O=2��CaSO4?2H2O����
�ʴ�Ϊ����1���ڢܣ�1��2��2����2��AD��CD����3��CaSO4? H2O����2CaSO4?H2O����2��CaSO4?
H2O����2CaSO4?H2O����2��CaSO4? H2O��+3H2O=2��CaSO4?2H2O��
H2O��+3H2O=2��CaSO4?2H2O��
��������1�����Ը��ݺ�������ˮ��Դ�ͷ�ֹ������Ⱦ������з������жϣ��Ӷ��ó���ȷ�Ľ��ۣ����ݻ�ѧ��Ӧǰ��ԭ�ӵ�������������������ƽ��
��2������H+��ˮ����γ�ˮ�������ӵ�ʵ����������
����������Ϣ��ˮʩ��һ�����糡���ڳ��¡���ѹ�£�ˮ���Խ�ɱ�����Ϊ���ȱ�������֪�ȱ��ܽ���̻����ȣ����б����������ʵȣ�
��3��������ʯ�����ʯ������������Լ�����֮����ת����ϵ���ش�
������������һ���ۺ��Ժ�ǿ����Ŀ��Ҫ��ѧ���Ӹ����Ƕ����ջ���֪ʶ���ѶȽϴ���Χ�ơ�ˮ����Ϊ���������龰���Ƚ����������뻯ѧ֪ʶ��ϵ������ͻ���˻�ѧSTSE�������ּ������������֪ʶ����������������ѧ��ʵ��Ӧ��֪ʶ���������Ⲣ���ѣ���Ҫ��ѧ������ȫ�沢�����ף��ر���ԡ��ȱ�������µĸ��Ҫ����ץסʵ����Ȼ��ˮ���ʲ���������ɱ��ͷ�ֹ�����ۻ�����;��
��2��H+��ˮ����γ�ˮ�������ӵķ���ʽΪ��H++H2O=H3O+�����������غ㶨�ɿ�֪��Ԫ������û�б仯��Ԫ�ػ��ϼ�Ҳû�б仯��������ɷ��Ӻ����ӽ�ϳ�Ϊ���Ӷ������仯����ѧ����Ҳ��Ϊ���ԣ�������ǰ�����������Ϊ10���ӣ��ޱ仯����ѡ��A��D��
�����ȱ��ĸ�����γ�������֪���ȱ�ʵ����Ȼ��ˮ���ʲ���������ɱ��ͷ�ֹ�����ۻ�����;�������ȱ����ص㡰�ڳ��¡���ѹ�£�ˮ���Խ�ɱ�����֪�������¿������������ܽ�������������ʴ�Ϊ��C��D��
��3��ҽҩ�Ͽ�����ʯ�����̶����۲�λ����ʯ����һ�����¶��º�ˮ��ϱ�Ϊ�ϴ�Ӳ�ȵ���ʯ�࣬�Ӷ��̶����ã�����ʽΪ��2��CaSO4?
 H2O��+3H2O=2��CaSO4?2H2O����
H2O��+3H2O=2��CaSO4?2H2O�����ʴ�Ϊ����1���ڢܣ�1��2��2����2��AD��CD����3��CaSO4?
 H2O����2CaSO4?H2O����2��CaSO4?
H2O����2CaSO4?H2O����2��CaSO4? H2O��+3H2O=2��CaSO4?2H2O��
H2O��+3H2O=2��CaSO4?2H2O����������1�����Ը��ݺ�������ˮ��Դ�ͷ�ֹ������Ⱦ������з������жϣ��Ӷ��ó���ȷ�Ľ��ۣ����ݻ�ѧ��Ӧǰ��ԭ�ӵ�������������������ƽ��
��2������H+��ˮ����γ�ˮ�������ӵ�ʵ����������
����������Ϣ��ˮʩ��һ�����糡���ڳ��¡���ѹ�£�ˮ���Խ�ɱ�����Ϊ���ȱ�������֪�ȱ��ܽ���̻����ȣ����б����������ʵȣ�
��3��������ʯ�����ʯ������������Լ�����֮����ת����ϵ���ش�
������������һ���ۺ��Ժ�ǿ����Ŀ��Ҫ��ѧ���Ӹ����Ƕ����ջ���֪ʶ���ѶȽϴ���Χ�ơ�ˮ����Ϊ���������龰���Ƚ����������뻯ѧ֪ʶ��ϵ������ͻ���˻�ѧSTSE�������ּ������������֪ʶ����������������ѧ��ʵ��Ӧ��֪ʶ���������Ⲣ���ѣ���Ҫ��ѧ������ȫ�沢�����ף��ر���ԡ��ȱ�������µĸ��Ҫ����ץסʵ����Ȼ��ˮ���ʲ���������ɱ��ͷ�ֹ�����ۻ�����;��

��ϰ��ϵ�д�
�����Ŀ
 ˮ������������Ȼ��Դ��û��ˮ��û�������������������յ��й�ˮ��֪ʶ�ش��������⣺
ˮ������������Ȼ��Դ��û��ˮ��û�������������������յ��й�ˮ��֪ʶ�ش��������⣺