��Ŀ����
(1)����ʳ�ؽ������ж���Ϊ���ᶾ�ԣ���÷������������е� ( )
A��ˮ B������ˮ C������ D����ˮ
(2)��ҽ������X�����θ��ʱ�����ò��˷������ᱵ(�׳Ʊ���)������Һ�����ᱵҲ���ؽ����Σ�Ϊʲô������û���˺�?______________________________��
(3)Ϊ�ⶨijBaCl2��Һ������������������ȡ100gBaCl2��Һ�����ϼ���ϡH2SO4����Ӧ��������Һ���������ϡH2SO4��������ϵ��ͼ��ʾ����ش�

��P������_______________��
�ڷ�Ӧ����BaSO4����������Ϊ_________g��
����ԭBaCl2��Һ����������������
(1) C (2) û�������ƶ��ı����ӻ�BaSO4������θ��
(3)����ȫ��Ӧ ��23.3 ��20.8%
��������
���������(1)�ؽ����ο���ʹ�����ʱ��ԣ��Ӷ��������ж������Լ��뵰�������ؽ����Σ�(2)�����������ؽ������ӣ��������к������ᱵû�������ƶ��ı����ӻ�BaSO4������θ�(3)��P����������Ȼ���ǡ����ȫ��Ӧ�ĵ㣬�ڷ�Ӧ����BaSO4����������Ϊ����100gϡ�������Һ�����ӵ�������Ϊ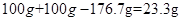 ��
��
�۽⣺��BaCl2����Ϊ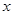
BaCl2+H2SO4=BaSO4 +2HCl
208 233
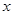 23.3g
23.3g
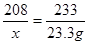
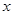
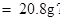
ԭBaCl2��Һ��������������Ϊ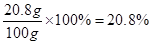
��ԭBaCl2��Һ����������������Ϊ20.8%
���㣺��ѧ����ʽ�ļ���
���������ǻ�ѧ��Ӧ��ϵ����ʽ�ļ����⣬������Ŀ��ÿ���п���ѹ���⣬�ؿ��⣬������Ŀ���ѵ����ڰ��������߱�ʾ������������ϵ������

(1)����ʳ�ؽ������ж���Ϊ���ᶾ�ԣ���÷������������е� ( )
| A��ˮ | B������ˮ | C������ | D����ˮ |
(3)Ϊ�ⶨijBaCl2��Һ������������������ȡ100gBaCl2��Һ�����ϼ���ϡH2SO4����Ӧ��������Һ���������ϡH2SO4��������ϵ��ͼ��ʾ����ش�
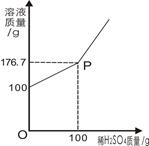
��P������_______________��
�ڷ�Ӧ����BaSO4����������Ϊ_________g��
����ԭBaCl2��Һ����������������
 ��2013?��������ģ����1������ʳ�ؽ������ж���Ϊ���ᶾ�ԣ���÷������������е�
��2013?��������ģ����1������ʳ�ؽ������ж���Ϊ���ᶾ�ԣ���÷������������е�