��Ŀ����
Ϊ̽��һƿ�������ƹ���ı��������ͬѧ�ǽ���������ʵ�飮
��ȡ�����ù�����Ʒ�����Թ��У������м���һ����ɫ��Һ�����������ݲ�����˵������Ʒ�к���̼���ƣ��ɴ˿�ȷ���ù����ѷ������ʣ�����ɫ��Һ������ ��
��Ϊ̽���ù������Ƿ���δ���ʵ��������ƣ�ͬѧ���ֽ��������±���ʾ��ʵ�飮��֪̼���Ƶ�ˮ��Һ�ʼ��ԣ����Ĵ��ڻ���������Ƶļ�����ɸ��ţ��������ͼ�������ʵ��ܽ��Ա���20�棩���ṩ����Ϣ��������д������
ʵ�����в��ֱ��ʵ��������ƹ��壮��ȡ�ù�����Ʒ10g������100gϡ�����У�ǡ����ȫ��Ӧ����Ӧ����Һ������Ϊ107.8g����
��1�����������������
��2������Ʒ���������Ƶ�����������
��ȡ�����ù�����Ʒ�����Թ��У������м���һ����ɫ��Һ�����������ݲ�����˵������Ʒ�к���̼���ƣ��ɴ˿�ȷ���ù����ѷ������ʣ�����ɫ��Һ������
��Ϊ̽���ù������Ƿ���δ���ʵ��������ƣ�ͬѧ���ֽ��������±���ʾ��ʵ�飮��֪̼���Ƶ�ˮ��Һ�ʼ��ԣ����Ĵ��ڻ���������Ƶļ�����ɸ��ţ��������ͼ�������ʵ��ܽ��Ա���20�棩���ṩ����Ϣ��������д������
| ������ ������ | OH- | NO3- | Cl- | SO42- | CO32- |
| H+ | �ܡ��� | �ܡ��� | �� | �ܡ��� | |
| Na+ | �� | �� | �� | �� | �� |
| Ba2+ | �� | �� | �� | ���� | ���� |
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
| ��ȥ̼���� | ȡ�����ù�����Ʒ�� ��ˮ�����Һ���μ������� | �а�ɫ�������� | �йط�Ӧ�Ļ�ѧ����ʽΪ |
| �����Ƿ��� �������� | ����Һ�еμӷ�̪��Һ | ����Ʒ�к����������� |
��1�����������������
��2������Ʒ���������Ƶ�����������
���㣺ҩƷ�Ƿ���ʵ�̽��,��Ļ�ѧ����,�εĻ�ѧ����,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��ѧ̽��
������������̼������������ɶ�����̼�����ʣ�����̼���ƵĴ��ڣ�
�ڳ�ȥ̼���ƣ����ǰ���������ת��Ϊ�����������ˮ����ȥ��֤���������Ʊ���ʱ��Ҫ����̼���Ƶ�ˮ��ҺҲ�Լ��ԣ�
��1��������Ŀ�е���Ϣ�����м������������������
��2�����ݶ�����̼�������ж�̼���Ƶ������������Ʒ���������Ƶ�������Ȼ���10g���бȽϼ�����ɽ��
�ڳ�ȥ̼���ƣ����ǰ���������ת��Ϊ�����������ˮ����ȥ��֤���������Ʊ���ʱ��Ҫ����̼���Ƶ�ˮ��ҺҲ�Լ��ԣ�
��1��������Ŀ�е���Ϣ�����м������������������
��2�����ݶ�����̼�������ж�̼���Ƶ������������Ʒ���������Ƶ�������Ȼ���10g���бȽϼ�����ɽ��
����⣺��̼���ο������ᷴӦ�ų���ʹ����ʯ��ˮ����ǵĶ�����̼���壻������ɫ��Һ�����ǣ�����ʴ�Ϊ�����ᣮ
���������ƹ���ı��ʻ�����̼���ƣ��������Ȼ�����Һ����̼���ƣ���Ϊ̼���ƺ��Ȼ�����Ӧ����̼�ᱵ��ɫ�������Ȼ��ƣ�̼�����Լ��ԣ���˼�����������ʱ��Ҫ���ų�̼���Ƶĸ��ţ����ȡ�����ù�����Ʒ����ˮ�����Һ���μ��������Ȼ�����Һ����ȥ̼���ƣ�Ȼ������Һ�еμӷ�̪��Һ��ֻҪ��ɫ���ɫ��֤�����������ƣ��ʴ�Ϊ���Ȼ�����Na2CO3+BaCl2=BaCO3��+2NaCl����ɫ���ɫ��
��1���������������Ϊ10g+100g-107.8g=2.2g
��2����̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
��
=
�����x=5.3g
��Ʒ���������Ƶ�����Ϊ��10g-5.3g=4.7g
��Ʒ���������Ƶ���������Ϊ��
��100%=47%
�ʴ�Ϊ��
�����ᣮ��
��1��2.2g����2��47%
���������ƹ���ı��ʻ�����̼���ƣ��������Ȼ�����Һ����̼���ƣ���Ϊ̼���ƺ��Ȼ�����Ӧ����̼�ᱵ��ɫ�������Ȼ��ƣ�̼�����Լ��ԣ���˼�����������ʱ��Ҫ���ų�̼���Ƶĸ��ţ����ȡ�����ù�����Ʒ����ˮ�����Һ���μ��������Ȼ�����Һ����ȥ̼���ƣ�Ȼ������Һ�еμӷ�̪��Һ��ֻҪ��ɫ���ɫ��֤�����������ƣ��ʴ�Ϊ���Ȼ�����Na2CO3+BaCl2=BaCO3��+2NaCl����ɫ���ɫ��
��1���������������Ϊ10g+100g-107.8g=2.2g
��2����̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
��
| 106 |
| x |
| 44 |
| 2.2g |
��Ʒ���������Ƶ�����Ϊ��10g-5.3g=4.7g
��Ʒ���������Ƶ���������Ϊ��
| 4.7g |
| 10g |
�ʴ�Ϊ��
�����ᣮ��
| ʵ��Ŀ�� | ʵ����� | ���� | ���ۻ�ѧ����ʽ |
| �Ȼ��� | Na2CO3+BaCl2=BaCO3��+2NaCl | ||
| ��ɫ���ɫ |
�����������ۺϿ������������Ƶ������Լ�̼����ļ��鷽�������ӷ�����ע����ռ����Լ����������ӹ����в��ܼ����µ��������ӣ���ȷѡ���Լ����ֿ����˻�ѧ����ʽ����д������ʵ����������ۣ��ۺ��ԱȽ�ǿ��ʵ��̽�����ǽ������п����ȵ�֮һ��������ʵ�鷽�����̵�̽����ʵ����ۺ�ʵ����ɵ�̽���ȣ�

��ϰ��ϵ�д�
�����Ŀ
ͼʾΪA��BԪ�ص�ԭ�ӽṹʾ��ͼ������˵��������ǣ�������
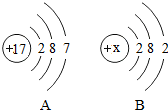

| A��B��ԭ�ӽṹʾ��ͼ��xΪ12 |
| B��A��B�ֱ����ڷǽ���Ԫ�غͽ���Ԫ�� |
| C��A��ԭ�Ӻ�B��ԭ�ӷֱ��γɼ����ӵĹ�����ͬ |
| D��A��B��ɵĻ������������ӹ��ɵ� |