��Ŀ����
11��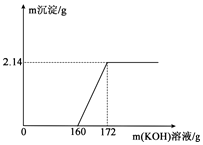 ��ҵ������ͨ������������FeCl3���ʻ�ɫ��Ϊ�˲ⶨij��ҵ������HCl�ĺ�����������ʵ�飬ͬѧȡij��ҵ����100g���μ�һ����������������KOH����ü���KOH��Һ�����뷴Ӧ���ɵij���������ϵ��ͼ��ʾ����ش��������⣺
��ҵ������ͨ������������FeCl3���ʻ�ɫ��Ϊ�˲ⶨij��ҵ������HCl�ĺ�����������ʵ�飬ͬѧȡij��ҵ����100g���μ�һ����������������KOH����ü���KOH��Һ�����뷴Ӧ���ɵij���������ϵ��ͼ��ʾ����ش��������⣺��1��FeCl3��KOHǡ����ȫ��Ӧʱ�����ɳ�����������2.14g��
��2�����KOH��Һ������������������д�����������̣�
��3����ù�ҵ������HCl������������������д�����������̣�
��4��ȡ100g�ù�ҵ����ϡ�ͳ���������Ϊ10%��ϡ���ᣬ�����ˮ����g����д�����������̣�
���� ��1������ͼ�е���Ϣ��֪���ɳ�����������
��2������������Ϣ��֪��Һ�����ԣ�������Ҫ���������ط�Ӧ�����ᷴӦ����Ȼ����ź��������ط�Ӧ�����ݳ������������÷�Ӧ�Ļ�ѧ����ʽ��������ĵ��������ص���������һ������KOH��Һ����������������
��3���������������������ᷴӦ�����Ȼ��ƺ�ˮ����������Ȼ����������
��4��������Һϡ��ǰ�����ʵ�����������
��� �⣺��1����ͼ��֪����ȫ��Ӧ�����ɳ���������Ϊ2.14g��
��2����ͼ��֪�����Ȼ�����Ӧ������������Һ������Ϊ172g-160g=12g��
�����ĵ��������ص�����Ϊx
FeCl3+3KOH�TFe��OH��3��+3KCl��
168 107
x 2.14g
$\frac{168}{x}=\frac{107}{2.14g}$
x=3.36g
����������Һ��������������=$\frac{3.36g}{12g}��$100%=28%��
��3�������ᷴӦ���������ص�����Ϊ��160g��28%=44.8g
HCl+KOH�TH2O+KCl��
36.5 56
y 44.8g
$\frac{36.5}{y}=\frac{56}{44.8g}$
y=29.2g
�ù�ҵ������HCl�����������ǣ�$\frac{29.2g}{100g}$��100%=29.2%
��4�����ˮ������Ϊz
100g��29.2%=��100g+z����10%
z=192g
�𰸣�
��1��2.14g��
��2����KOH��Һ��������������28%��
��3���ù�ҵ������HCl������������29.2%��
��4�����ˮ������Ϊ192g��
���� ������һ���ۺ��Լ����⣬����Ҫ������������Ϣ����Һ�ijɷ֣������Ҫ����ͼ������ȡ��Ϣ����������ѧ֪ʶ���ݻ�ѧ����ʽ���м��㣮

| A�� | �õ�ȼ�ķ�����ȥO2�е�H2��H2+O2$\frac{\underline{\;��ȼ\;}}{\;}$H2O | |
| B�� | �ô����ʯ������ȡ�������ռNa2C03+Ca��OH��2�TCaC03��+2NaOH | |
| C�� | �������ȥNa2SO4�е�Na2 CO3��2HCl+Na2C03�T2NaCl+H2 O+C02�� | |
| D�� | �����۳�ȥFeSO4�е�CuSO4��2Fe+3CuSO4�TFe2��S04��3+3Cu |
| A�� | ���� NaOH �������� | |
| B�� | ��ʯ�� CaO ������� | |
| C�� | С�մ�NaHCO3 ���ͷ���Ҫ�ɷ�֮һ | |
| D�� | �ɱ�CO2 �˹����� |
| A�� | �������֬���ṩ���� | B�� | ������ë����˿����Ȼ��ά | ||
| C�� | ȱ��������ƶѪ�� | D�� | ������CaO������ʯ�� |
 ���������ѧ��Ǯ��������ɫӫ�⵰���о���Ӧ�÷����ͻ���������2008��ŵ������ѧ�����ʰ��ᣨC2H5NO2���������ɫӫ�⵰��һ�ֳɷ֣�ͬʱҲ������DZ����һ�ְ����ᣮ
���������ѧ��Ǯ��������ɫӫ�⵰���о���Ӧ�÷����ͻ���������2008��ŵ������ѧ�����ʰ��ᣨC2H5NO2���������ɫӫ�⵰��һ�ֳɷ֣�ͬʱҲ������DZ����һ�ְ����ᣮ