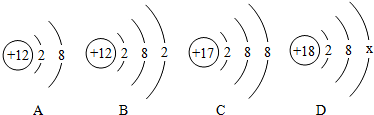
题目内容
某同学为确定一敞口放置的氢氧化钠溶液的变质情况,分别取50g溶液于在个烧杯中,分别向其中滴加几滴酚酞溶液,再向其中分别滴加溶质质量分数为7.3%的稀盐酸,使其充分反应,有关变化的现象和数据如下表格:
请回答下列问题:
(1)写出实验中发生反应的化学方程式 ;
(2)第一次实验后的溶液中溶质的化学式为 ;
(3)根据已知条件列出求解50g溶液中所含碳酸钠质量(x)的比例式 ;
(4)若向第三次反应后的滤液中加入154.4g水,则所得不饱和溶液中溶质的质量分数为 ;
(5)原样品中变质的氢氧化钠与末变质的氢氧化钠的质量比为 .
| 第一次 | 第二次 | 第三次 | |
| 盐酸质量/g | 50 | 150 | 200 |
| 烧杯内剩余物质的质量/g | 100 | 197.8 | 245.6 |
| 现象 | 无气泡,溶液仍为红色 | 溶液中有气泡产生,溶液仍为红色 | 溶液中有气泡产生,溶液恰好由红色变为无色 |
(1)写出实验中发生反应的化学方程式
(2)第一次实验后的溶液中溶质的化学式为
(3)根据已知条件列出求解50g溶液中所含碳酸钠质量(x)的比例式
(4)若向第三次反应后的滤液中加入154.4g水,则所得不饱和溶液中溶质的质量分数为
(5)原样品中变质的氢氧化钠与末变质的氢氧化钠的质量比为
考点:根据化学反应方程式的计算,有关溶质质量分数的简单计算
专题:综合计算(图像型、表格型、情景型计算题)
分析:敞口放置的氢氧化钠溶液能与空气中的二氧化碳反应生成了碳酸钠和水,在加入盐酸时,碳酸钠、氢氧化钠与盐酸发生了反应.由质量守恒定律可知,向50g溶液中加入200g的稀盐酸,溶液恰好由红色变为无色,说明了加入的稀盐酸恰好与氢氧化钠和碳酸钠反应,混合物减少的就是生成的二氧化碳的质量,由二氧化碳的质量可求出碳酸钠的质量、生成的氯化钠的质量,与碳酸钠反应的盐酸的质量,由此可求出与氢氧化钠反应的盐酸的质量,求出氯化钠的质量,再根据溶质的质量分数求出不饱和溶液中溶质的质量分数等.
解答:解:生成二氧化碳的质量为:50g+200g-245.6g=4.4g
设溶液中碳酸钠的质量为x、生成的氯化钠的质量为y,与碳酸钠反应的稀盐酸中溶质的质量为z
Na2CO3+2HCl=2NaCl+H2O+CO2↑
106 73 117 44
x z y 4.4g
=
=
=
解得:x=10.6g z=7.3g y=11.7g
与碳酸钠反应的盐酸的质量为:
=100g,
与氢氧化钠反应的盐酸的质量为:200g-100g=100g.
设氢氧化钠的质量为q,生成的氯化钠的质量为p
HCl+NaOH=NaCl+H2O
36.5 40 58.5
100g×7.3% q p
=
=
解得:q=8g p=11.7g
(1)由上分析可知,实验中发生反应的化学方程式是:HCl+NaOH=NaCl+H2O,Na2CO3+2HCl=2NaCl+H2O+CO2↑;
(2)由上计算可知,与氢氧化钠反应的盐酸的质量为100g,所以,在第一次实验是加入的盐酸的质量为50g,溶液中的氢氧化钠未完全反应,反应后的溶液中溶质的化学式为:NaCl、NaOH;
(3)由上述计算可知,50g溶液中所含碳酸钠质量(x)的比例式为:
=
;
(4)若向第三次反应后的滤液中加入154.4g水,则所得不饱和溶液中溶质的质量分数为:
×100%=5.85%;
(5)设变质的氢氧化钠的质量为w,
CO2+2NaOH═Na2CO3+H2O
80 106
w 10.6g
=
解得:w=8g
原样品中变质的氢氧化钠与末变质的氢氧化钠的质量比为:8g:8g=1:1.
故答为:(1)HCl+NaOH=NaCl+H2O,Na2CO3+2HCl=2NaCl+H2O+CO2↑(2)NaCl、NaOH;(3)
=
;(4)5.85%;(5)1:1.
设溶液中碳酸钠的质量为x、生成的氯化钠的质量为y,与碳酸钠反应的稀盐酸中溶质的质量为z
Na2CO3+2HCl=2NaCl+H2O+CO2↑
106 73 117 44
x z y 4.4g
| 106 |
| x |
| 73 |
| z |
| 117 |
| y |
| 44 |
| 4.4g |
解得:x=10.6g z=7.3g y=11.7g
与碳酸钠反应的盐酸的质量为:
| 7.3g |
| 7.3% |
与氢氧化钠反应的盐酸的质量为:200g-100g=100g.
设氢氧化钠的质量为q,生成的氯化钠的质量为p
HCl+NaOH=NaCl+H2O
36.5 40 58.5
100g×7.3% q p
| 36.5 |
| 100g×7.3% |
| 40 |
| q |
| 58.5 |
| p |
解得:q=8g p=11.7g
(1)由上分析可知,实验中发生反应的化学方程式是:HCl+NaOH=NaCl+H2O,Na2CO3+2HCl=2NaCl+H2O+CO2↑;
(2)由上计算可知,与氢氧化钠反应的盐酸的质量为100g,所以,在第一次实验是加入的盐酸的质量为50g,溶液中的氢氧化钠未完全反应,反应后的溶液中溶质的化学式为:NaCl、NaOH;
(3)由上述计算可知,50g溶液中所含碳酸钠质量(x)的比例式为:
| 106 |
| 44 |
| x |
| 4.4g |
(4)若向第三次反应后的滤液中加入154.4g水,则所得不饱和溶液中溶质的质量分数为:
| 11.7g+11.7g |
| 245.6g+154.4g |
(5)设变质的氢氧化钠的质量为w,
CO2+2NaOH═Na2CO3+H2O
80 106
w 10.6g
| 80 |
| 106 |
| w |
| 10.6g |
原样品中变质的氢氧化钠与末变质的氢氧化钠的质量比为:8g:8g=1:1.
故答为:(1)HCl+NaOH=NaCl+H2O,Na2CO3+2HCl=2NaCl+H2O+CO2↑(2)NaCl、NaOH;(3)
| 106 |
| 44 |
| x |
| 4.4g |
点评:本题的综合性较强,涉及的知识点较多,难度较大,加强化学方程式的书写、有关溶质质量分数、化学方程式的计算是解答本题的关键.

练习册系列答案
相关题目
自然界的水因含有许多杂质而需要净化,下列操作不能使水得到净化的是( )
| A、吸附 | B、电解 | C、过滤 | D、蒸馏 |
下列有关除杂质所用的试剂和操作方法中,不正确的是( )
| 序号 | 物质 | 杂质 | 试剂 | 操作方法 |
| A | 盐酸 | 硫酸 | 适量的氯化钡溶液 | 过滤 |
| B | 氯化钙 | 碳酸钙 | 适量的稀盐酸 | 蒸发、结晶 |
| C | 二氧化碳 | 水蒸气 | 浓硫酸 | 洗气 |
| D | 硝酸钠溶液 | 碳酸钠 | 适量的氯化钙溶液 | 过滤 |
| A、A | B、B | C、C | D、D |
下列化学方程式书写正确的是( )
| A、2Fe+3H2SO4═Fe2(SO4)3+3H2↑ | ||||
| B、Cu+AgNO3═Cu(NO3)2+Ag | ||||
C、CO+CuO
| ||||
| D、CaCO3+2HCl═CaCl2+H2O+CO2 |
乙烷是重要的化工原料,根据乙烷分子结构模型(如图),下列叙述错误的是( )
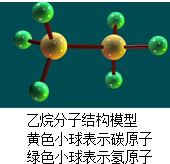

| A、乙烷由两种元素组成 | ||
| B、乙烷中碳、氢元素的质量之比为12:1 | ||
| C、乙烷分子中碳、氢原子个数之比为1:3 | ||
D、乙烷中氢元素的质量分数为
|