��Ŀ����
ij��ѧ��ȤС��ͬѧΪ�ⶨпͭ�Ͻ���п��������������ȡ8.0�˺Ͻ���Ʒ����60.0��ϡ�����6�μ�����Ʒ�У���ַ�Ӧ����ˡ�ϴ�ӡ�����������õ�ʵ���������£�
| ϡH2SO4���� | ʣ��������� |
| ��һ�μ���10.0�� | m |
| �ڶ��μ���10.0�� | 5.4�� |
| �������10.0�� | 4.1�� |
| ���Ĵμ���10.0�� | 2.8�� |
| ������10.0�� | 1.6�� |
| �������10.0�� | 1.6�� |
��2����пͭ�Ͻ�п����������Ϊ________��
��3����������ϡH2SO4�����ʵ���������������д��������̣������ȷ��0.1%��
�⣺��1�����ݱ����е����ݿɷ�����10��ϡ������1.3��пǡ����ȫ��Ӧ����m��ֵΪ��8g-1.3g=6.7g��
�ʴ�Ϊ��6.7g��
��2����Ϊͭ�������ᷴӦ�����ʣ��Ĺ���ijɷ���ͭ������пͭ�Ͻ���п������Ϊ��8g-1.6g=6.4g
пͭ�Ͻ���п����������Ϊ ��100%=80%�� �ʴ�Ϊ��80%��
��100%=80%�� �ʴ�Ϊ��80%��
��3��ÿ10.0gϡ������1.3gпǡ����ȫ��Ӧ
��10.0gϡ���������ʵ�����Ϊx
Zn+H2SO4�TZnSO4+H2��
65 98
1.3g x
 =
= ��� x=1.96g
��� x=1.96g
ϡ���������ʵ�������Ϊ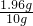 ��100%=19.6%��
��100%=19.6%��
��������1�����ݱ����е����ݿɷ�������10��ϡ����ǡ����ȫ��Ӧ��þ��������������ȷ��m��ֵ��
��2����Ӧǰ��������ʼ��ٵ�����Ϊп������������ ��100%�����пͭ�Ͻ���п������������
��100%�����пͭ�Ͻ���п������������
��3�����ݱ��������ݷ�����֪10��ϡ������1.3��пǡ����ȫ��Ӧ������п�����ᷴӦ�Ļ�ѧ����ʽ������п���������10��ϡ���������ʵ�������Ȼ����� ��100%�����ϡ���������ʵ�����������
��100%�����ϡ���������ʵ�����������
������������һ���йر���������ۺϼ����⣬�Ȿ��Ĺؼ��Ǹ��ݱ����е����ݷ������μӷ�Ӧ���������ʵ�������ϵ��
�ʴ�Ϊ��6.7g��
��2����Ϊͭ�������ᷴӦ�����ʣ��Ĺ���ijɷ���ͭ������пͭ�Ͻ���п������Ϊ��8g-1.6g=6.4g
пͭ�Ͻ���п����������Ϊ
 ��100%=80%�� �ʴ�Ϊ��80%��
��100%=80%�� �ʴ�Ϊ��80%����3��ÿ10.0gϡ������1.3gпǡ����ȫ��Ӧ
��10.0gϡ���������ʵ�����Ϊx
Zn+H2SO4�TZnSO4+H2��
65 98
1.3g x
 =
= ��� x=1.96g
��� x=1.96gϡ���������ʵ�������Ϊ
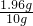 ��100%=19.6%��
��100%=19.6%����������1�����ݱ����е����ݿɷ�������10��ϡ����ǡ����ȫ��Ӧ��þ��������������ȷ��m��ֵ��
��2����Ӧǰ��������ʼ��ٵ�����Ϊп������������
 ��100%�����пͭ�Ͻ���п������������
��100%�����пͭ�Ͻ���п��������������3�����ݱ��������ݷ�����֪10��ϡ������1.3��пǡ����ȫ��Ӧ������п�����ᷴӦ�Ļ�ѧ����ʽ������п���������10��ϡ���������ʵ�������Ȼ�����
 ��100%�����ϡ���������ʵ�����������
��100%�����ϡ���������ʵ�����������������������һ���йر���������ۺϼ����⣬�Ȿ��Ĺؼ��Ǹ��ݱ����е����ݷ������μӷ�Ӧ���������ʵ�������ϵ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
ij��ѧ��ȤС��ͬѧΪ�ⶨþͭ�Ͻ���þ��������������ȡ3.0g�Ͻ���Ʒ����60.0gϡ�����6�μ�����Ʒ�У���ַ�Ӧ����ˡ�ϴ�ӡ�������أ��õ���ʵ���������£�
��1���ⶨ�����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��������m= g��
��3����þͭ�Ͻ���þ����������Ϊ ��
��4����������ϡ���������ʵ����������� ����д��������̣������ȷ��0.1%��
| ϡ�������� | ʣ��������� |
| ��һ�μ���10.0g | m |
| �ڶ��μ���10.0g | 2.0g |
| �������10.0g | 1.5g |
| ���Ĵμ���10.0g | 1.0g |
| ������10.0g | 0.6g |
| �������10.0g | 0.6g |
��2��������m=
��3����þͭ�Ͻ���þ����������Ϊ
��4����������ϡ���������ʵ�����������
