��Ŀ����
ij��ȤС���߽�ʵ���ҿ�����һ������г�ġ�����������ͼ1����

��1������˾��������뵽��ȡ�ú�Ӧ��������ƿ���ܷⱣ�棬������Ϊ �����û�ѧ����ʽ����˵����
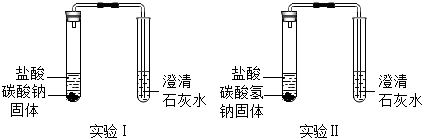
��2������ȤС��ͬѧΪ�˲ⶨ���ʵ��ռ���Ʒ��Na2CO3���������������������ͼ2��ʾ��װ�ã�������������ᷴӦ��ͨ���Ҳ�ע�������ռ���������������Na2CO3��������������ʹ��ע�����������Ϊ20mL����
д����ƿ���������仯�Ļ�ѧ����ʽ �� ��С����Ϊ�÷�������Ҫ������Ʒȡ�õ�����������Ϊ ��
��3������ȤС��ͬѧ�ڷ�˼����ʵ����������ͼ3��װ�òⶨ���ʵ��ռ���Ʒ��Na2CO3��������������Ӧ�Ŀ�ʼ��������Ҫ��ע�������������Ϳ�����ʵ�鲽��Ϊ��
�ټ��װ�õ������ԣ�
�ڳ�10g�ռ���Ʒ������ƿ�У�D��ע�����ڼ���������ϡ���ᣬȷ����װ��F������320g������װ�ã�
�۴��ɼУ���������ע����A 10�Σ�ȷ����װ��F������320.5g��
�ܹرյ��ɼУ�Ȼ���ƶ�ע����M�Ļ�������ϡ������ε�����Ʒ�У�ֱ�����ٲ�������Ϊֹ��
�ݴ��ɼУ���������ע����A 10�Σ�����ȷ��װ��F����Ϊ321.6g��
����̽����
�������������ע����A�������ʹ��죬��ⶨ��Na2CO3������������ ���ƫ����ƫС���������䡱���������� ��
�����Ը���ʵ���������ݼ�������ռ���Ʒ��Na2CO3���������� ��������д��������̣�

��1������˾��������뵽��ȡ�ú�Ӧ��������ƿ���ܷⱣ�棬������Ϊ
��2������ȤС��ͬѧΪ�˲ⶨ���ʵ��ռ���Ʒ��Na2CO3���������������������ͼ2��ʾ��װ�ã�������������ᷴӦ��ͨ���Ҳ�ע�������ռ���������������Na2CO3��������������ʹ��ע�����������Ϊ20mL����
д����ƿ���������仯�Ļ�ѧ����ʽ
��3������ȤС��ͬѧ�ڷ�˼����ʵ����������ͼ3��װ�òⶨ���ʵ��ռ���Ʒ��Na2CO3��������������Ӧ�Ŀ�ʼ��������Ҫ��ע�������������Ϳ�����ʵ�鲽��Ϊ��
�ټ��װ�õ������ԣ�
�ڳ�10g�ռ���Ʒ������ƿ�У�D��ע�����ڼ���������ϡ���ᣬȷ����װ��F������320g������װ�ã�
�۴��ɼУ���������ע����A 10�Σ�ȷ����װ��F������320.5g��
�ܹرյ��ɼУ�Ȼ���ƶ�ע����M�Ļ�������ϡ������ε�����Ʒ�У�ֱ�����ٲ�������Ϊֹ��
�ݴ��ɼУ���������ע����A 10�Σ�����ȷ��װ��F����Ϊ321.6g��
����̽����
�������������ע����A�������ʹ��죬��ⶨ��Na2CO3������������
�����Ը���ʵ���������ݼ�������ռ���Ʒ��Na2CO3����������
���㣺ҩƷ�Ƿ���ʵ�̽��,��������ļ�������ӷ���,��Ļ�ѧ����,�εĻ�ѧ����,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��ѧ̽��
��������1���������ƹ��������տ����е�ˮ�ֶ����⣬���������̼��Ӧ��д����Ӧ�ķ���ʽ��
��2����֪��ij���ʵ��ռ���Ʒ����Na2CO3�������Ժ����ᷴӦ�Ļ�ѧ����ʽӦ������������ʵ���Ŀ�ļ������Ϣ������
��3��������������̼�ͷŵ�̫�죬���ܱ�����������ȫ���գ���Ӱ��ⶨ��Na2CO3���������������ݴ��ɼ�1��ȷ����װ��F������320.5�˺�321.6�ˣ���������Ϊ������̼��������֪��������̼����������ɸ��ݻ�ѧ����ʽ�����̼���Ƶ��������Ӷ�������ռ���Ʒ��Na2CO3������������
��2����֪��ij���ʵ��ռ���Ʒ����Na2CO3�������Ժ����ᷴӦ�Ļ�ѧ����ʽӦ������������ʵ���Ŀ�ļ������Ϣ������
��3��������������̼�ͷŵ�̫�죬���ܱ�����������ȫ���գ���Ӱ��ⶨ��Na2CO3���������������ݴ��ɼ�1��ȷ����װ��F������320.5�˺�321.6�ˣ���������Ϊ������̼��������֪��������̼����������ɸ��ݻ�ѧ����ʽ�����̼���Ƶ��������Ӷ�������ռ���Ʒ��Na2CO3������������
����⣺��1���������ƹ��������տ����е�ˮ�ֶ����⣬���������̼��Ӧ����̼���ƺ�ˮ�����ʣ���Ӧ�ķ���ʽΪ��2NaOH+CO2�TNa2CO3+H2O�����Ҫ�ܷⱣ�棻
��2����֪��ij���ʵ��ռ���Ʒ����Na2CO3�������Ժ����ᷴӦ�Ļ�ѧ����ʽӦ�����������������ƺ�̼����������ķ�Ӧ����Ӧ�ķ���ʽ�ֱ�Ϊ��NaOH+HCl=NaCl+H2O��Na2CO3+2HCl=2NaCl+H2O+CO2������Ϊ��ʹ��ע�����������Ϊ20mL������Ʒȡ���ˣ�����������ܳ���ע�������̣�����Ʒȡ���ˣ���������̫�٣��������������Ҫ������Ʒȡ�õ�����
��3��������������̼�ͷŵ�̫�죬���ܱ�����������ȫ���գ���ʹ�ⶨ��Na2CO3����������ƫС��
���⣺���������غ㶨�ɿ�֪����������̼������=321.6g-320.5g=1.1g��
����Ʒ��̼���Ƶ�����Ϊx��
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106 44
X 1.1g
=
X=2.65g
��Ʒ��̼���Ƶ���������Ϊ��
�w100%=26.5%
�𣺸��ռ���Ʒ��Na2CO3����������Ϊ26.5%��
�ʴ�Ϊ����1��2NaOH+CO2�TNa2CO3+H2O��
��2��NaOH+HCl�TNaCl+H2O��Na2CO3+2HCl�T2NaCl+H2O+CO2��������Ʒȡ���ˣ�����������ܳ���ע�������̣�����Ʒȡ���ˣ���������̫�٣���������
��3����ƫС�������ǣ����ɶ�����̼���岻����ȫ��NaOH��Һ���գ�
��26.5%��
��2����֪��ij���ʵ��ռ���Ʒ����Na2CO3�������Ժ����ᷴӦ�Ļ�ѧ����ʽӦ�����������������ƺ�̼����������ķ�Ӧ����Ӧ�ķ���ʽ�ֱ�Ϊ��NaOH+HCl=NaCl+H2O��Na2CO3+2HCl=2NaCl+H2O+CO2������Ϊ��ʹ��ע�����������Ϊ20mL������Ʒȡ���ˣ�����������ܳ���ע�������̣�����Ʒȡ���ˣ���������̫�٣��������������Ҫ������Ʒȡ�õ�����
��3��������������̼�ͷŵ�̫�죬���ܱ�����������ȫ���գ���ʹ�ⶨ��Na2CO3����������ƫС��
���⣺���������غ㶨�ɿ�֪����������̼������=321.6g-320.5g=1.1g��
����Ʒ��̼���Ƶ�����Ϊx��
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106 44
X 1.1g
| 106 |
| 44 |
| X |
| 1.1g |
��Ʒ��̼���Ƶ���������Ϊ��
| 2.65g |
| 10g |
�𣺸��ռ���Ʒ��Na2CO3����������Ϊ26.5%��
�ʴ�Ϊ����1��2NaOH+CO2�TNa2CO3+H2O��
��2��NaOH+HCl�TNaCl+H2O��Na2CO3+2HCl�T2NaCl+H2O+CO2��������Ʒȡ���ˣ�����������ܳ���ע�������̣�����Ʒȡ���ˣ���������̫�٣���������
��3����ƫС�������ǣ����ɶ�����̼���岻����ȫ��NaOH��Һ���գ�
��26.5%��
������������Ҫ̽��ҩƷ�Ƿ���ʣ�����̽�����ʵ���ɳɷ��Լ������ķ�����ѧ��ʵ�����ݴ��������ķ������ص��ǣ����ո��ݻ�ѧ����ʽ����IJ���ͷ�����

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д�
�����Ŀ