��Ŀ����
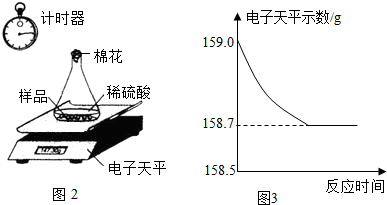
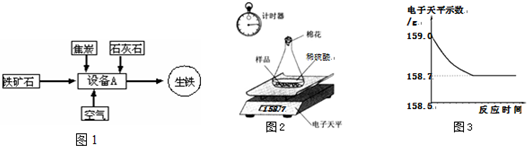
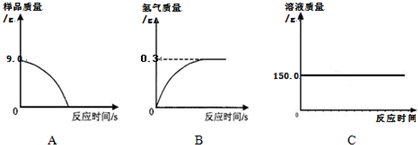
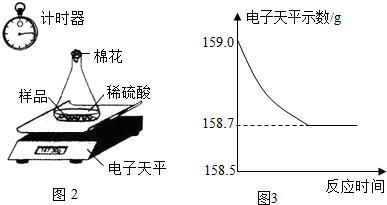
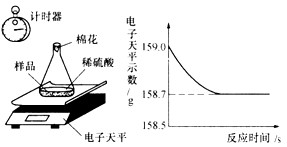
С��ͬѧ�ɼ���һЩ������Ʒ�������ʣ����ʲ�����ˮ������ϡ���ᷴӦ������������ͼ��ʾװ�ý��з������ֱ�Ƶ���ƿ����������Ϊ44.1g��������Ʒ������Ϊ9.0g������ƿ�м�������ϡ�����������ʼ��¼������ƽ��ʾ������¼��������ͼ��ʾ��
������������ݣ��ش��������⣺
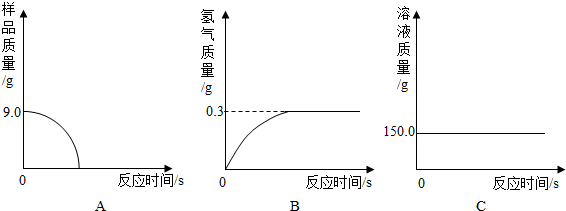
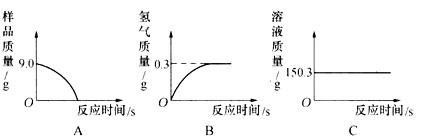
(1)С�����ݴ���������ͼ������ȷ����__________��
(1)С�����ݴ���������ͼ������ȷ����__________��
(2)������Ʒ������������������д�����̣���
(3)���㷴Ӧ��������Һ������������������������д�����̣���
(3)���㷴Ӧ��������Һ������������������������д�����̣���
(1)B
(2)�⣺�跴Ӧ����������Ϊx�����ɵ���������������Ϊy��
Fe+H2SO4==FeSO4+H2��
56����������152��2
x������������y��0.3g
�ó�x=8.4g��������������Ϊ8.4g/9g= 93. 3%
y=22.8g
(3)����������������������Ϊ22.8g��(8.4g+159.0g-9.0g-44.lg-0.3g)��100%=20%
(2)�⣺�跴Ӧ����������Ϊx�����ɵ���������������Ϊy��
Fe+H2SO4==FeSO4+H2��
56����������152��2
x������������y��0.3g
�ó�x=8.4g��������������Ϊ8.4g/9g= 93. 3%
y=22.8g
(3)����������������������Ϊ22.8g��(8.4g+159.0g-9.0g-44.lg-0.3g)��100%=20%

��ϰ��ϵ�д�
�����Ŀ