��Ŀ����
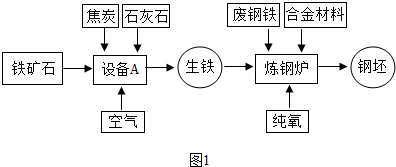
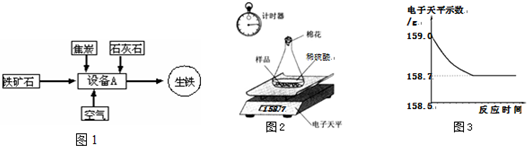
������ҵ��һ�����ҵ�֧����ҵ֮һ��ͼ1�ǹ�ҵ�����Ļ�����������ʾ��ͼ��
��ش��������⣺

��1��д���豸A �����ƣ�______��
��2��д���Գ�����Ϊԭ���ڸ�������ȡ���Ļ�ѧ����ʽ______��
��3��ʯ��ʯ����Ҫ������������д���ڸ���������ʯ��ʯ�ֽ�Ļ�ѧ��Ӧ����ʽ��______��
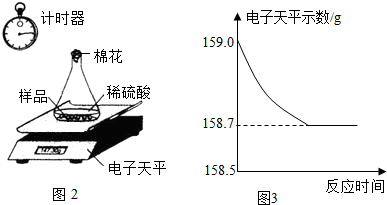
��4��С��ͬѧ�ɼ���һЩ������Ʒ�������ʣ����ʲ�����ˮ������ϡ���ᷴӦ��������ͼ2��ʾװ�ý��з������ֱ�Ƶ���ƿ����������Ϊ44.1g��������Ʒ������Ϊ9.0g������ƿ�м�������ϡ�����������ʼ��¼������ƽ��ʾ������¼������
ͼ3��
������������ݣ��ش��������⣺
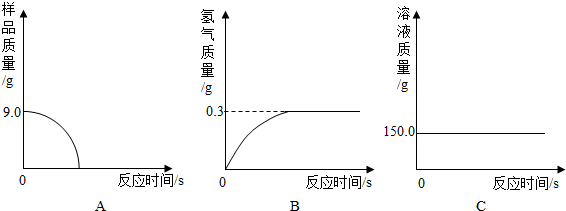
��5��С�����ݴ�����ͼ������ȷ����______

��6��������Ʒ������������������д�����̣�
�⣺��1����ҵ������ʹ�õ��豸Ϊ��¯�������������ڸ�¯����ɵģ�
��2���������е��������ڸ�������һ����̼����������ԭ��Ӧ�����ɵ����������������̼������ʽΪ��3CO+Fe2O3 2Fe+3CO2��
2Fe+3CO2��
��3��ʯ��ʯ����Ҫ�ɷ�̼����ڸ��¶���ʱ�ֽ����������ƺͶ�����̼������ʽΪ��CaCO3 CaOʮCO2����
CaOʮCO2����
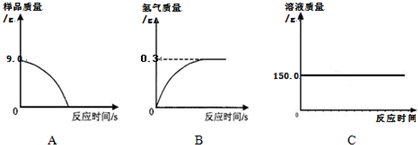
��5��������������Ʒ�е����ʲ�����ˮ�Ҳ����ᷢ����Ӧ����˷�Ӧֹͣ����Ʒ��������Ϊ0������ͼA��ʾ�ķ�������
��������������ϡ���ᷴӦ���ų�������������������ȫ��Ӧ���ų�����������=159.0g-158.7g=0.3g������ͼB�ķ�����ȷ��
����ϡ���������=159.0g-44.1g-9.0g=105.9g����Ӧʹ��Һ������������ֱ����Ӧֹͣ��Һ�������ٸı䣻����ͼC�ķ�������
��6������Ʒ�е���������Ϊx
Fe+H2SO4�TFeSO4+H2��
56 2
x 0.3g
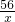 =
=
��� x=8.4g
������������= ��100%��93.3%
��100%��93.3%
�ʴ�Ϊ����1����¯��
��2��3CO+Fe2O3 2Fe+3CO2��
2Fe+3CO2��
��3��CaCO3 CaOʮCO2����
CaOʮCO2����
��5��B��
��6����Ʒ��������������Ϊ93.3%��
��������1����ҵ�����ֳ�Ϊ��¯��������Ϊ��ҵ����ʹ�õ��豸Ϊ��¯��
��2��������ʯ�е��������ڸ�������һ����̼��Ӧ���������Ͷ�����̼��
��3��̼��Ƹ��¶��տɷֽ����������ƺͶ�����̼��
��5�����ݷ�Ӧ��¼���ݣ���ȫ��Ӧ�ų���������=159.0g-158.7g=0.3g������ϡ���������=159.0g-44.1g-9.0g=105.9g������Ʒ�������������Ӧ�����жϣ�
��6����Ϸ�Ӧ�Ļ�ѧ����ʽ����������������������������������������ɷ����ͼ��㣮
���������������ķ�Ӧԭ��������ʽ����ķ������������������ͼ��仯����д����ʽ�ȣ��ܺܺõĿ���ѧ���������֪ʶ�����ͽ�������������
��2���������е��������ڸ�������һ����̼����������ԭ��Ӧ�����ɵ����������������̼������ʽΪ��3CO+Fe2O3
 2Fe+3CO2��
2Fe+3CO2����3��ʯ��ʯ����Ҫ�ɷ�̼����ڸ��¶���ʱ�ֽ����������ƺͶ�����̼������ʽΪ��CaCO3
 CaOʮCO2����
CaOʮCO2������5��������������Ʒ�е����ʲ�����ˮ�Ҳ����ᷢ����Ӧ����˷�Ӧֹͣ����Ʒ��������Ϊ0������ͼA��ʾ�ķ�������
��������������ϡ���ᷴӦ���ų�������������������ȫ��Ӧ���ų�����������=159.0g-158.7g=0.3g������ͼB�ķ�����ȷ��
����ϡ���������=159.0g-44.1g-9.0g=105.9g����Ӧʹ��Һ������������ֱ����Ӧֹͣ��Һ�������ٸı䣻����ͼC�ķ�������
��6������Ʒ�е���������Ϊx
Fe+H2SO4�TFeSO4+H2��
56 2
x 0.3g
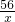 =
=
��� x=8.4g
������������=
 ��100%��93.3%
��100%��93.3%�ʴ�Ϊ����1����¯��
��2��3CO+Fe2O3
 2Fe+3CO2��
2Fe+3CO2����3��CaCO3
 CaOʮCO2����
CaOʮCO2������5��B��
��6����Ʒ��������������Ϊ93.3%��
��������1����ҵ�����ֳ�Ϊ��¯��������Ϊ��ҵ����ʹ�õ��豸Ϊ��¯��
��2��������ʯ�е��������ڸ�������һ����̼��Ӧ���������Ͷ�����̼��
��3��̼��Ƹ��¶��տɷֽ����������ƺͶ�����̼��
��5�����ݷ�Ӧ��¼���ݣ���ȫ��Ӧ�ų���������=159.0g-158.7g=0.3g������ϡ���������=159.0g-44.1g-9.0g=105.9g������Ʒ�������������Ӧ�����жϣ�
��6����Ϸ�Ӧ�Ļ�ѧ����ʽ����������������������������������������ɷ����ͼ��㣮
���������������ķ�Ӧԭ��������ʽ����ķ������������������ͼ��仯����д����ʽ�ȣ��ܺܺõĿ���ѧ���������֪ʶ�����ͽ�������������

��ϰ��ϵ�д�
�����Ŀ