��Ŀ����
��ij���������֪�û�����п��ܺ���FeCl3��NaCl��NH4NO3��CuSO4���������е����ֻ���֣�Ϊ̽������ɣ���������ʵ�飨����������з����ķ�Ӧ��ǡ����ȫ��Ӧ����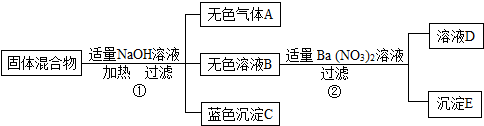
�Ը���ʵ����̺ͷ�����������д���¿հף�
��1����ʪ��ĺ�ɫʯ����ֽ��������A����ֽ����ɫ��A�Ļ�ѧʽΪ��
��2������������������������У��϶������ڵ������� ��д��ѧʽ����
��3������ҺD�У�һ�����е��������ǣ�д���ӷ��ţ�
��4������������������������У�������ȷ�����ڵ������ǣ�д��ѧʽ�� ��

�Ը���ʵ����̺ͷ�����������д���¿հף�
��1����ʪ��ĺ�ɫʯ����ֽ��������A����ֽ����ɫ��A�Ļ�ѧʽΪ��
��2������������������������У��϶������ڵ�������
��3������ҺD�У�һ�����е��������ǣ�д���ӷ��ţ�
��4������������������������У�������ȷ�����ڵ������ǣ�д��ѧʽ��
���㣺���ʵļ����ƶ�,��������ļ�������ӷ���,�εĻ�ѧ����
ר�⣺�������ɵ��ƶ���
�����������̬���ʺͼ��ϻ�ų�������������ʹʪ��ĺ�ɫʯ����Һ����������ͭ���������ƻ�������ɫ��������ͭ�����������Ӻ���������ӡ�̼������ӻ����ɳ���������������Һ���Ի�ɫ��֪ʶ���з�����
����⣺�̬���ʺͼ��ϻ�ų�������������ʹʪ��ĺ�ɫʯ����Һ����������ͭ���������ƻ�������ɫ��������ͭ�����������Ӻ���������ӡ�̼������ӻ����ɳ���������������Һ���Ի�ɫ��
��1���̬�����мӼ�����ɰ�������������ˮ�Լ��ԣ�������ʹ��ɫ��ʯ����ֽ����ɫ������A�Ļ�ѧʽ�ǣ�NH3��
��2�����������������Ȼ�����Ӧ���ɺ��ɫ�����������������Ȼ��ƣ���ͼʾ��֪û�к��ɫ�������ɣ�˵���������Ȼ�����
��3����ɫ��ҺB�м������������ᱵ������������뱵�����������ᱵ��������������ҺD�У�һ�����ڵ�����������������ӣ�
��4�����������������������ɣ�˵��ԭ������к���笠����ӣ�����������泥�����ɫ��������˵������ͭ���ӣ�����������ͭ��û�к��ɫ�������ɣ�˵���������Ȼ��������Բ���ȷ�������Ȼ��ƣ�
�ʴ�Ϊ����1��NH3��
��2��FeCl3��
��3��NO3-��
��4��NaCl��
��1���̬�����мӼ�����ɰ�������������ˮ�Լ��ԣ�������ʹ��ɫ��ʯ����ֽ����ɫ������A�Ļ�ѧʽ�ǣ�NH3��
��2�����������������Ȼ�����Ӧ���ɺ��ɫ�����������������Ȼ��ƣ���ͼʾ��֪û�к��ɫ�������ɣ�˵���������Ȼ�����
��3����ɫ��ҺB�м������������ᱵ������������뱵�����������ᱵ��������������ҺD�У�һ�����ڵ�����������������ӣ�
��4�����������������������ɣ�˵��ԭ������к���笠����ӣ�����������泥�����ɫ��������˵������ͭ���ӣ�����������ͭ��û�к��ɫ�������ɣ�˵���������Ȼ��������Բ���ȷ�������Ȼ��ƣ�
�ʴ�Ϊ����1��NH3��
��2��FeCl3��
��3��NO3-��
��4��NaCl��
�������ڽ������ʱ�����ȷ��������������ʵ����ʣ�Ȼ���������е�����ȷ���������ʵĴ����ԣ����ȷ�������ijɷ֣�

��ϰ��ϵ�д�
 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д� Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д�
�����Ŀ
�û�ѧ�Ĺ۵����������еij������ʣ�ijͬѧ�ó������¼������ۣ����д��ڴ�����ǣ�������
| A�����Ϸ����Ǽ��ֻ��ʵĻ���� |
| B�������pH��5.6 |
| C��������Ǽ������� |
| D�����õ�ú��Һ��ʯ�������ǻ�ʯȼ�� |
��˿��������ȼ�յ���Ҫʵ�������ǣ�������
| A�������������� |
| B��ȼ�յĻ���Ϊ��ɫ |
| C���������䣬�к�ɫ�������� |
| D�������д̼�����ζ������ |
 �ס������ֹ������ʵ��ܽ��������ͼ��ʾ���ֽ���֧�ֱ�װ�мס��������ʱ�����Һ���Թܣ��ײ���������δ�ܽ�Ĺ��壩����ʢ��ˮ���ձ��У������ձ��м���һ����Ũ���ᣮ
�ס������ֹ������ʵ��ܽ��������ͼ��ʾ���ֽ���֧�ֱ�װ�мס��������ʱ�����Һ���Թܣ��ײ���������δ�ܽ�Ĺ��壩����ʢ��ˮ���ձ��У������ձ��м���һ����Ũ���ᣮ ����������κ�ˮ�ķ�Ӧ���кͷ�Ӧ��ʵ����ѧ��ѧС���ͬѧ���кͷ�Ӧ�Ƿ���Ⱥ��кͷ�Ӧ�������������й���������̽����
����������κ�ˮ�ķ�Ӧ���кͷ�Ӧ��ʵ����ѧ��ѧС���ͬѧ���кͷ�Ӧ�Ƿ���Ⱥ��кͷ�Ӧ�������������й���������̽����