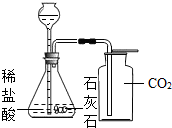
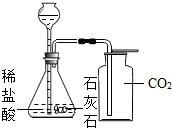
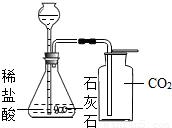
��Ŀ����
С��ͬѧΪ�ⶨʯ��ʯ��̼��Ƶ��������������ʲ����ᷴӦ������6.0gʯ��ʯ��Ʒ����μ���ϡ���ᣨ��������������5%��15%�������ٲ�������Ϊֹ�������ɶ�����̼����2.2g���Լ��㣺CaCO3+2HCl�TCaCl2+H2O+CO2��
��1����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣���д��������̣������ȷ��0.1%��
��2����Ҫ����������Ӧ��������������������㲹��һ��������______����������������Ϊ��______g��
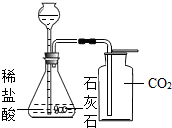
��1����ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣���д��������̣������ȷ��0.1%��
��2����Ҫ����������Ӧ��������������������㲹��һ��������______����������������Ϊ��______g��

�����ɶ�����̼2.2g��Ҫ̼��Ƶ�����Ϊx������HCl����Ϊy
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 44
x y 2.2g
=
x=5g
=
y=3.65g
��1����ʯ��ʯ��Ʒ��̼��Ƶ���������=
��100%=83.3%
�𣺸�ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ83.3%��
��2��������Һ��������������=
��100%������֪��Һ����������Ҫ����Һ����������Ҫ��֪��Һ�����ʵ���������������������ϣ�ϡ�������������Ϊ10%����Ӧ���������������=3.65g��10%=36.5g
�ʴ�Ϊ��ϡ�������������Ϊ10%��36.5��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 44
x y 2.2g
| 100 |
| x |
| 44 |
| 2.2g |
| 73 |
| y |
| 44 |
| 2.2g |
��1����ʯ��ʯ��Ʒ��̼��Ƶ���������=
| 5g |
| 6.0g |
�𣺸�ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ83.3%��
��2��������Һ��������������=
| �������� |
| ��Һ���� |
�ʴ�Ϊ��ϡ�������������Ϊ10%��36.5��

��ϰ��ϵ�д�
�����Ŀ