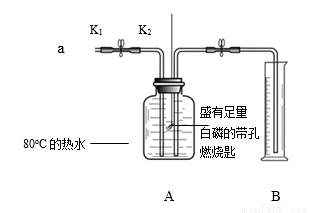
��Ŀ����
��ҵ�ϣ�ͨ������ת�����Ƶ�KClO3���塣

��1��KClO3����Ԫ�صĻ��ϼ�Ϊ_______��
��2������������NaCl��Һ�ɴ���ˮ���ƶ��ɣ�����ʱ��ȥ����ˮ����ɳ�����õIJ�����_______��
��3�����Ƣ��з�Ӧ�Ļ�ѧ����ʽ��NaCl+3H2O=NaClO3 +3_______
��4����֪NaClO3+KCl=NaCl+KClO3������������������Һ��KClO3��_______���������Һ����������Һ������
��5�����������У���ѭ�����õ�������_______
��ϰ��ϵ�д�
�����Ŀ
�������ʵ���������;�Ķ�Ӧ��ϵ������ǣ�������
ѡ�� | ���� | ��; |
A | �������Ƴʼ��� | ������������ |
B | Ũ���������ˮ�� | �������� |
C | ϡ��������ijЩ���������ﷴӦ | ������ |
D | ������������ijЩ�ǽ��������ﷴӦ | ���ն������� |
A. A B. B C. C D. D
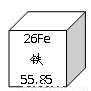
��ͼΪԪ�����ڱ���4���ڵ�һ���֡��ݴ��ж�����˵���д������( )
26 Fe �� 55.85 | 27 Co �� 58.93 | 28 Ni �� 58.69 | 29 Cu ͭ 63.55 |
A. ��Ԫ�صķ���ΪN i B. ��Ԫ�ص����ԭ��������58.93g
C. ��Ԫ�ض����ڽ���Ԫ�� D. �����Ҹ�Ԫ�ص�ԭ��������������