��Ŀ����
��1��Ҫ��78.3g��̼���ƹ��壬����ʱ���������ŷ��ˣ�5g���������룬��Ƶ���̼���ƹ����ʵ������Ϊ ��
��2����ȡˮ�ȸ��Ӷ���Ϊ38mL�����������Ӷ���Ϊ15mL����ʵ������ˮ 23mL��������ڡ���С�ڡ����ڡ���ȷ������
��3��С��ͬѧ����ҽ���Ľ�̸�л�Ϥ��������ÿ������Ҫ����8L�������ʳ�����ÿ���Ӵ�����Ҫ�������������ڱ�״���£�8L�����������Ƕ��٣���O2���ܶ�Ϊ1.429��/�������� д��������̣��������һλС����
��2����ȡˮ�ȸ��Ӷ���Ϊ38mL�����������Ӷ���Ϊ15mL����ʵ������ˮ
��3��С��ͬѧ����ҽ���Ľ�̸�л�Ϥ��������ÿ������Ҫ����8L�������ʳ�����ÿ���Ӵ�����Ҫ�������������ڱ�״���£�8L�����������Ƕ��٣���O2���ܶ�Ϊ1.429��/�������� д��������̣��������һλС����
���㣺������-������ƽ,��������-��Ͳ,�����ijɷּ����ɷֵ��������
ר�⣺������������ѧʵ���������
��������1��������ƽ��ʹ�÷������������룬��֪���̵������������̵����������������������������=��������+��������������Ŵ��ˣ���Ҫ�������̵�����=���̵�����+�����������ֻ��������������λ�ý����ˣ��е�ʽ���м��㣮
��2��������Ͳ��ʹ�÷��������Ӻ��Ӳ������ľ���������н��⣬���Ӷ���ƫ�ߣ����Ӷ���ƫ�ͣ�
��3�������и��ɷֵ���������ֱ��ǣ�������Լռ78%��������Լռ21%��ϡ�������Լռ0.94%��������̼��Լռ0.03%��ˮ������������������ʴ�Լռ0.03%���йصļ���Ҫ��ȷ��
��2��������Ͳ��ʹ�÷��������Ӻ��Ӳ������ľ���������н��⣬���Ӷ���ƫ�ߣ����Ӷ���ƫ�ͣ�
��3�������и��ɷֵ���������ֱ��ǣ�������Լռ78%��������Լռ21%��ϡ�������Լռ0.94%��������̼��Լռ0.03%��ˮ������������������ʴ�Լռ0.03%���йصļ���Ҫ��ȷ��
����⣺��1�������̵�����=���̵�����+�����������֪����������=��������+�����������������������=��������-����������������������=75g-3.3g=71.7g��
�ʴ�Ϊ��71.7g��
��2�����Ӱ�Һ�����ʹ�ʱ�������Ķ���ƫ����ȡ��ʵ���������ƫС���������Ϊ38mL��ʵ��С��38mL�����Ӱ�Һ�����ʹ��������Ķ���ƫС������ȡ��ʵ��Һ��ƫ���ɰ�Һ�����ʹ�����Ϊ15mL��ʵ�ʱ�15mL���������Һ������С��23mL��
�ʴ�Ϊ��С�ڣ�
��3�������и��ɷֵ���������ֱ��ǣ�������Լռ78%��������Լռ21%��������ÿ������Ҫ����8L������������ÿ���Ӵ�����Ҫ���������=8L��21%��38.1L���ڱ�״���£�8L������������=8L��1.429g/L��11.4g���𣺳�����ÿ���Ӵ�����Ҫ����38.1L���ڱ�״���£�8L������������11.4g��
�ʴ�Ϊ��38.1L��11.4g��
�ʴ�Ϊ��71.7g��
��2�����Ӱ�Һ�����ʹ�ʱ�������Ķ���ƫ����ȡ��ʵ���������ƫС���������Ϊ38mL��ʵ��С��38mL�����Ӱ�Һ�����ʹ��������Ķ���ƫС������ȡ��ʵ��Һ��ƫ���ɰ�Һ�����ʹ�����Ϊ15mL��ʵ�ʱ�15mL���������Һ������С��23mL��
�ʴ�Ϊ��С�ڣ�
��3�������и��ɷֵ���������ֱ��ǣ�������Լռ78%��������Լռ21%��������ÿ������Ҫ����8L������������ÿ���Ӵ�����Ҫ���������=8L��21%��38.1L���ڱ�״���£�8L������������=8L��1.429g/L��11.4g���𣺳�����ÿ���Ӵ�����Ҫ����38.1L���ڱ�״���£�8L������������11.4g��
�ʴ�Ϊ��38.1L��11.4g��
�������ڼ����й���ƽ����Ŀʱ��Ҫ�������̵������T���̵�����+������������ɴ��е�ʽ���㣬�������������뻹����������������㣮

��ϰ��ϵ�д�
 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
�����Ŀ
����˵���У�����ȷ���ǣ�������
| A����������ͷ���Ͻ��ʯ�����������ò��� |
| B���������������˶��������û���̿���������� |
| C������ú��й©���ŵ�һ�����ŵ����壬����CO���� |
| D�������δ�����IJ˽�֮ǰ���������ƻ����� |
���������У��仯ѧʽ�����������������ȷ���ǣ�������
| A��̼��� CaCO3 ������ |
| B������ H2SO4 ������ |
| C������������ N2O5������ |
| D������ Cl2 ���� |

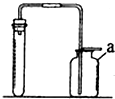 ��ͼ��ʵ������ȡ����ij���װ�ã���ش��������⣮
��ͼ��ʵ������ȡ����ij���װ�ã���ش��������⣮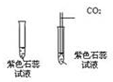 ��֤������̼������ʵ�飺
��֤������̼������ʵ�飺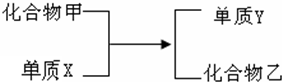 ��������뵥��X��һ�������·�Ӧ�����ɵ���Y����ɫ���壩�ͻ������ң���ͼ��ʾ�����������趨�������£��ƶ�X������ʲô���ʣ����ѧʽ��
��������뵥��X��һ�������·�Ӧ�����ɵ���Y����ɫ���壩�ͻ������ң���ͼ��ʾ�����������趨�������£��ƶ�X������ʲô���ʣ����ѧʽ��