��Ŀ����
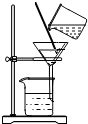
10��ͼ1��ijͬѧ��ɡ�ʵ��5 һ�������������Ȼ�����Һ�����ơ��С�����50g��������Ϊ6%���Ȼ�����Һ����ȫ���̣�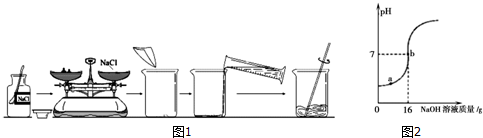
��1��ͼ1�еĴ����У���ҩƷƿ���������������ϣ��������ҩƷλ�õߵ���
��2���������Ƶ���ˮ��NaCl ����������С��6%��������������ԭ���Тټ����д�����ƽ��������ȷ���������Ȼ����������ʣ��ձ���ԭ����ˮ��ˮ�����ˣ���ƽδ���㣩
��3��Ҫ��50g��������Ϊ98%��Ũ����ϡ��Ϊ��������Ϊ20%�����ᣬ��Ҫˮ�������ǣ���ʵ������Ũ��������ϡ�������Ҫ�����У����㡢��ȡ�����ȡ���ȴ������װƿ�����ϱ�ǩ��
��4��ȡijϡ������Ʒ10g����5%��NaOH��Һ��μ��뵽��Ʒ�У��ӱ߽��裮��ҺpH�ı仯��ͼ2��ʾ���Իش�
��a����Һ�к��е�������Na+��H+��SO42��
�ڵ�pH=7ʱ������NaOH��Һ��NaOH������Ϊ0.8g��
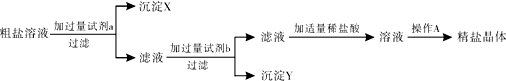
�ۼ���ϡ���������������������д��������̣�
���� ��1��ȡ�ù���ҩƷʱ��ƿ��Ӧ���ţ�������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ�ݴ˽��з������
��2����������Һ����������С����Ҫ���Ƶ�6%���ɴ˿��ж������Ƶ���Һ�������Ȼ�������ƫС���ܼ�ˮ����ƫ���µĽ������ˣ��ڷ����������ԭ��ʱ��ӦΧ����������ԭ���������������IJ�����
��3����ˮϡ��Ũ��Һ��ϡ��Һ�Ĺ����У����ʵ��������䣬��Һ������=��Һ���ܶȡ���Һ��������ݴ��е�ʽ���㣬����������Һ�IJ���������
��4����a����ʾ��Һ�����ԣ�
�ڸ������ʵ�����=��Һ����������Һ�����ʵ��������������㣻
�۸����������Ƶ����������û�ѧ����ʽ��������������������һ�������ϡ���������ʵ�����������
��� �⣺��1����ȡ�ù���ҩƷʱ��Ϊ��ֹ��ȾҩƷ��ƿ��Ӧ���ţ�ͼ��ƿ��û�е��ţ�
��������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ��ͼ���������Ȼ��Ƶ�λ�÷ŷ��ˣ�
��2����������Һ����������С����Ҫ���Ƶ�6%���ɴ˿��ж������Ƶ���Һ�������Ȼ�������ƫС���ܼ�ˮ����ƫ���µĽ�������ܵ�ԭ���ǣ����ټ����д����Ȼ��Ʒ�������ƽ�����̣�������ƽ��������ȷ�������Ȼ����������ʣ����ձ���ԭ����ˮ����ˮ�����ˣ�����ƽδ���㣻����ת���Ȼ���ʱ�Ȼ��������������ϣ�
��3����������ǰ����Һ�е������������䣬����Ҫ����ˮ������Ϊx�����У�50g��98%=��50g+x����20%�����x=195g����ʵ������Ũ��������ϡ�������Ҫ�����У����㡢��ȡŨ�����ˮ��Ȼ����ȡ���ȴ������װƿ�����ϱ�ǩ��
��4����a���Ӧ����Һ�����ԣ���������ʣ�࣬��Һ�����ʷֱ�Ϊ�����ƺ����ᣬ���ڵ������������ӡ���������Ӻ������ӣ�
������NaOH��Һ��NaOH������Ϊ��16g��5%=0.8g��
����10gϡ�����к���H2SO4������Ϊx
H2SO4+2NaOH�TNa2SO4+2H2O
98 80
x 0.8g
$\frac{98}{x}=\frac{80}{0.8g}$
x=0.98g
ϡ�����������������Ϊ$\frac{0.98g}{10g}$��100%=9.8%��
�ʴ�Ϊ��
��1����ҩƷƿ���������������ϣ� �������ҩƷλ�õߵ���
��2���ټ����д�����ƽ��������ȷ���������Ȼ����������ʣ��ձ���ԭ����ˮ�� ˮ�����ˣ� ��ƽδ���㣩��
��3��195g����ȡ��
��4����Na+��H+��SO42-�� ��0.8����ϡ�����������������Ϊ9.8%��
���� �����ѶȲ�����ȷ����һ������������������Һʵ�鲽�衢ע����д���ݻ�ѧ����ʽ�ļ��������ȷ�����Ĺؼ���

| A�� | ������֧��ȼ�գ����������ȼ�ϵ���ȼ�� | |
| B�� | ̼�����������ᷴӦ������������θ����� | |
| C�� | ����������ʱ�ų�������������Ϊ���幩�� | |
| D�� | �������ƹ�������ˮ������������CO2���� |
| A�� | Cu+AgNO3�TCu��NO3��2+Ag | B�� | 2Fe+6HCl�T2FeCl3+3H2�� | ||
| C�� | 2H2+O2$\frac{\underline{\;��ȼ\;}}{\;}$2H2O | D�� | 2KMnO4�TK2MnO4+MnO2+O2�� |
| A�� | ����ֻ�������������Ļ�ѧ��Ӧ����������Ӧ | |
| B�� | ͨ�������ڿ�����ȼ��ʵ��������������������������Ӧ | |
| C�� | ��������������һ�����ʵķ�Ӧ���ǻ��Ϸ�Ӧ | |
| D�� | ���������ڿ����ж���ȼ�� |
| A�� | ��Ԫ�� | B�� | ��Ԫ�� | C�� | ̼Ԫ�� | D�� | ��Ԫ�� |
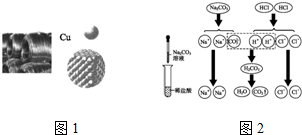

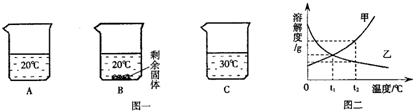
 ij������Ʒ�к��������Ȼ�þ���Ȼ��ƣ�С������������ᴿ������
ij������Ʒ�к��������Ȼ�þ���Ȼ��ƣ�С������������ᴿ������