��Ŀ����
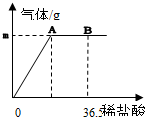 �ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g�����������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺
�ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g�����������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺��1��A��������������m=
��2��������̼���Ƶ����������������ȷ��0.1%����
��3��B��ʱ���ձ�����Һ�е�������
��������1�����ݲ�����������������ϡ�����������ϵͼ��A��ʱ̼����������ǡ����ȫ��Ӧ��֮���ټ�ϡ����Ϊ�������ٷ�����Ӧ����ˣ����������غ㶨�ɣ����������ձ�����Һ�������뷴Ӧǰ���Ӹ������������IJ�ֵ������÷ų�������̼������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ����ǡ����ȫ��Ӧ�ų�������̼���������ɼ�����Ʒ��������̼���Ƶ�����������������Ʒ�����ȿɼ���������̼���Ƶ�����������
��3����A��ǡ����ȫ��Ӧ������ϡ��������Ͳ��ٷ�����Ӧ����ˣ���B��ʱ�����������������ҺΪ�Ȼ��ƺ�ϡ����Ļ����Һ��
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ����ǡ����ȫ��Ӧ�ų�������̼���������ɼ�����Ʒ��������̼���Ƶ�����������������Ʒ�����ȿɼ���������̼���Ƶ�����������
��3����A��ǡ����ȫ��Ӧ������ϡ��������Ͳ��ٷ�����Ӧ����ˣ���B��ʱ�����������������ҺΪ�Ȼ��ƺ�ϡ����Ļ����Һ��
����⣺��1�����������غ㶨�ɣ�A��������������=36.5g+6g-40.3g=2.2g��
�ʴ�Ϊ��2.2g��
��2������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
=
x=5.3g
������̼���Ƶ���������=
��100%=88.3%
��������̼���Ƶ���������Ϊ88.3%��
��3��B��ʱ����ϡ���������������ҺΪ�Ȼ�����ϡ����Ļ����Һ����������ΪNaCl��HCl��
�ʴ�Ϊ��NaCl��HCl��
�ʴ�Ϊ��2.2g��
��2������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 2.2g
| 106 |
| x |
| 44 |
| 2.2g |
������̼���Ƶ���������=
| 5.3g |
| 6g |
��������̼���Ƶ���������Ϊ88.3%��
��3��B��ʱ����ϡ���������������ҺΪ�Ȼ�����ϡ����Ļ����Һ����������ΪNaCl��HCl��
�ʴ�Ϊ��NaCl��HCl��
������������ʾ��Ӧ���ɵ�����ʱ�����ߵ��۵㼴ͼ��A��Ϊǡ����ȫ��Ӧ���˺�����ϡ������������ٷ�����Ӧ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g�����������������ȫ���ݳ�����������������������ϡ�����������ϵ����ͼ��ʾ���Լ��㣺
�ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g�����������������ȫ���ݳ�����������������������ϡ�����������ϵ����ͼ��ʾ���Լ��㣺 �ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣬ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g���������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺
�ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣬ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g���������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺 ��2011?������ģ�⣩�ҹ�����ij�κ������Ĵ����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������������������ϡ�����������ϵ����ͼ��ʾ���������������ȫ���ݳ�����������μ���ͼA��ʱ���ձ�����Һ��������Ϊ40.3g�����Լ��㣺
��2011?������ģ�⣩�ҹ�����ij�κ������Ĵ����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������������������ϡ�����������ϵ����ͼ��ʾ���������������ȫ���ݳ�����������μ���ͼA��ʱ���ձ�����Һ��������Ϊ40.3g�����Լ��㣺 ��2013?��������ģ���ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������Һ����������μ���CaCl2��Һ�������������ʵ���������CaCl2����������ϵ��ͼ��ʾ��
��2013?��������ģ���ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������Һ����������μ���CaCl2��Һ�������������ʵ���������CaCl2����������ϵ��ͼ��ʾ��