��Ŀ����
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�Na+��NH4+��Ba2+��Cl-��CO32-��SO42-����ȡ����200mL��Һ��������ʵ�飺�ٵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������0.68g���ڵڶ��ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g����������ʵ�飬�����Ʋ���ȷ����Aһ��������Ba2+��NH4+���ܴ��� B��CO32-һ������
C��Na+һ�������� D����
���𰸡���������Һ�е�NH4+�����������ƻ�ų�������CO32-��Ba2+�����γɲ�����ˮ��������ϡ�����̼�ᱵ��ɫ������SO42-��Ba2+�����γɲ�����ˮҲ��������ϡ��������ᱵ��ɫ��������ˣ�CO32-��SO42-��Ba2+������ͬһ��Һ�й��森
����⣺�ɵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������0.68g�ɶ϶���Һ��һ�����а������ӣ��ɵڶ�����Һ��ʵ�飬�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g���ɵ���Һ��һ������̼������Ӻ������������һ�������ڱ����ӣ�
���ݸ��������ݣ������Һ���ֵ����Կ��Լ��㣺
笠�����Ϊ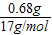 =0.04mol
=0.04mol
���������Ϊ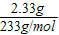 =0.01mol
=0.01mol
̼�������Ϊ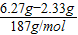 =0.02mol
=0.02mol
���������0.01mol×2+0.02×2=0.06mol
��笠����������0.04mol×1=0.04mol������㷨���ֽ̲������ἰ��
����Ӧ�û��������ӣ����������Ѿ�ȷ������������һ�����������ӣ�
������������Һ��һ������Na+��NH4+��CO32-��SO42-���ӣ�һ������Ba2+���ӣ����ܺ���Cl-���ӣ�
��ѡB��
Dѡ���Ϊ��һ������Na+��NH4+��SO42-���ӣ�
������������Һ�����Ӽ�Ĺ����ϵ���ڽ�����������кܴ��������Һ�е����Ӳ����γ����塢ˮ�ͳ���ʱ�����ӿ��Թ��森
����⣺�ɵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������0.68g�ɶ϶���Һ��һ�����а������ӣ��ɵڶ�����Һ��ʵ�飬�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g���ɵ���Һ��һ������̼������Ӻ������������һ�������ڱ����ӣ�
���ݸ��������ݣ������Һ���ֵ����Կ��Լ��㣺
笠�����Ϊ
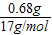 =0.04mol
=0.04mol���������Ϊ
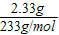 =0.01mol
=0.01mol̼�������Ϊ
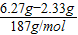 =0.02mol
=0.02mol���������0.01mol×2+0.02×2=0.06mol
��笠����������0.04mol×1=0.04mol������㷨���ֽ̲������ἰ��
����Ӧ�û��������ӣ����������Ѿ�ȷ������������һ�����������ӣ�
������������Һ��һ������Na+��NH4+��CO32-��SO42-���ӣ�һ������Ba2+���ӣ����ܺ���Cl-���ӣ�
��ѡB��
Dѡ���Ϊ��һ������Na+��NH4+��SO42-���ӣ�
������������Һ�����Ӽ�Ĺ����ϵ���ڽ�����������кܴ��������Һ�е����Ӳ����γ����塢ˮ�ͳ���ʱ�����ӿ��Թ��森

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
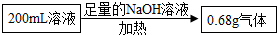 �ɴ˼��㣺200mL��Һ�к�NH4+������Ϊ
�ɴ˼��㣺200mL��Һ�к�NH4+������Ϊ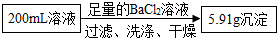 �ɴ˼��㣺200mL��Һ�к�CO32-������Ϊ
�ɴ˼��㣺200mL��Һ�к�CO32-������Ϊ