摘要:10.25℃.101kPa下.碳.氢气.甲烷和葡萄糖的燃烧热依次是393.5kJ/mol.285.8kJ/mol.890.3kJ/mol.2800kJ/mol.则下列热化学方程式正确的是 A.C (a) + CO (g),△H = -393.5 kJ/ mol B.2H2 (g) + O2 (g) 2H2O (g),△H = + 571.6 kJ/ mol C.CH4 (g) + 2O2 (g) CO 2 (g) +2H2O (g),△H = -890.3 kJ/ mol D.C6H12O6 (g) +3O2 (g) 3CO2 (g)+3H2O (I); △H = -1400 kJ/ mol
网址:http://m.1010jiajiao.com/timu3_id_47084[举报]
在25℃、101kPa下,白磷(化学式为P4)、红磷(化学式为P)燃烧的热化学方程式分别为:
P4(s)+5O2(g)=P4O10(s);△H=-3093.2kJ?mol-1
4P(s)+5O2(g)=P4O10(s);△H=-2954.0kJ?mol-1
由此判断下列说法正确的是( )
P4(s)+5O2(g)=P4O10(s);△H=-3093.2kJ?mol-1
4P(s)+5O2(g)=P4O10(s);△H=-2954.0kJ?mol-1
由此判断下列说法正确的是( )
查看习题详情和答案>>
25℃、101kPa下:
①2Na(s)+
O2(g)═Na2O(s)△H1=-414kJ/mol;
②2Na(s)+O2(g)═Na2O2(s)△H2=-511kJ/mol.
下列说法正确的是( )
①2Na(s)+
| 1 |
| 2 |
②2Na(s)+O2(g)═Na2O2(s)△H2=-511kJ/mol.
下列说法正确的是( )
| A、①和②生成等物质的量的产物,转移的电子数相同 |
| B、Na与足量O2反应生成Na2O,随温度升高生成Na2O的速率逐渐加快 |
| C、25℃、101 kPa下,Na2O2(s)+2Na(s)═2Na2O(s)△H=+317 kJ/mol |
| D、①和②产物中的阴阳离子个数比均为1:1 |
能源问题是人类社会面临的重大课题.甲醇是未来重要的绿色能源之一.
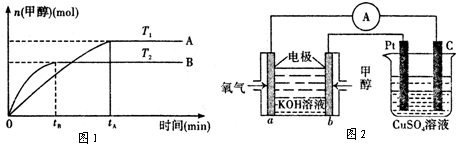
(l)已知:在 25℃、101kPa 下,1g 甲醇燃烧生成 CO2和液态水时放热 22.70kJ.请写出甲醇燃烧的热化学方程式 .
(2)由CO2和H2合成甲醇的化学方程式为:CO2(g)+3H2(g)
CH3OH(g)+H2O(g)在其它条件不变的情况下,实验测得温度对反应的影响如图1所示(注:T1、T2均大于300℃):
①合成甲醇反应的△H 0.(填“>”、“<”或“=”).
②平衡常数的表达式为: .温度为T2时的平衡常数 温度为T1时的平衡常数(填“>”、“<”或“=”)
③在T1温度下,将1mol CO2和 1molH2充入一密闭恒容容器中,充分反应达到平衡后,若CO2转化率为α,则容器内的压强与起始压强的比值为 .
(3)利用甲醇燃料电池设计如图2所示的装置.该装置中 Pt 极为 极;写出b极的电极反应式 .
查看习题详情和答案>>

(l)已知:在 25℃、101kPa 下,1g 甲醇燃烧生成 CO2和液态水时放热 22.70kJ.请写出甲醇燃烧的热化学方程式
(2)由CO2和H2合成甲醇的化学方程式为:CO2(g)+3H2(g)
| 催化剂 | 加热 |
①合成甲醇反应的△H
②平衡常数的表达式为:
③在T1温度下,将1mol CO2和 1molH2充入一密闭恒容容器中,充分反应达到平衡后,若CO2转化率为α,则容器内的压强与起始压强的比值为
(3)利用甲醇燃料电池设计如图2所示的装置.该装置中 Pt 极为
写出下列热化学方程式
(1)已知25℃、101kpa下,1gC8H18(辛烷)燃烧生成二氧化碳和液态水时放出48.40kJ热量.写出25℃、101kPa时的辛烷燃烧热的热化学方程式
(2)在一定条件下,N2和H2完全反应生成1molNH3放热46.0kJ热量.写出氨分解为氢气和氮气的热化学方程式
查看习题详情和答案>>
(1)已知25℃、101kpa下,1gC8H18(辛烷)燃烧生成二氧化碳和液态水时放出48.40kJ热量.写出25℃、101kPa时的辛烷燃烧热的热化学方程式
C8H18(l)+
O2(g)=8CO2(g)+9H2O(g)△H=-5517.6KJ/mol
| 25 |
| 2 |
C8H18(l)+
O2(g)=8CO2(g)+9H2O(g)△H=-5517.6KJ/mol
.| 25 |
| 2 |
(2)在一定条件下,N2和H2完全反应生成1molNH3放热46.0kJ热量.写出氨分解为氢气和氮气的热化学方程式
2NH3(g)=N2(g)+3H2(g)△H=+92KJ/mol
2NH3(g)=N2(g)+3H2(g)△H=+92KJ/mol
.利用盖斯定律解答下列各小题
(1)已知:TiO2(s)+2Cl2(g)═TiCl4(l)+O2(g)△H=+140kJ?mol-1
2C(s)+O2(g)═2CO(g)△H=-221kJ?mol-1
写出TiO2和焦炭、氯气反应生成TiCl4和CO气体的热化学方程式:
(2)25℃、101kPa下:①2Na(s)+
O2(g)═Na2O(s)△H1=-414kJ?mol-1
②2Na(s)+O2(g)═Na2O2(s)△H2=-511kJ?mol-1
写出该条件下由Na2O2和Na生成Na2O的热化学方程式:
(3)已知:C(s,石墨)+O2(g)═CO2(g)△H1=-393.5kJ?mol-1;
2H2(g)+O2(g)═2H2O(l)△H2=-571.6kJ?mol-1;
2C2H2(g)+5O2(g)═4CO2(g)+2H2O(l)△H2=-2599kJ?mol-1;
写出由C(s,石墨)和H2(g)生成1mol C2H2(g)的热化学方程式
查看习题详情和答案>>
(1)已知:TiO2(s)+2Cl2(g)═TiCl4(l)+O2(g)△H=+140kJ?mol-1
2C(s)+O2(g)═2CO(g)△H=-221kJ?mol-1
写出TiO2和焦炭、氯气反应生成TiCl4和CO气体的热化学方程式:
2C(s)+TiO2(s)+2Cl2(g)═TiCl4(l)+2CO(g)△H=-81kJ?mol-1
2C(s)+TiO2(s)+2Cl2(g)═TiCl4(l)+2CO(g)△H=-81kJ?mol-1
.(2)25℃、101kPa下:①2Na(s)+
| 1 | 2 |
②2Na(s)+O2(g)═Na2O2(s)△H2=-511kJ?mol-1
写出该条件下由Na2O2和Na生成Na2O的热化学方程式:
2Na(s)+Na2O2(s)=2Na2O(s)△H1=-317kJ?mol-1
2Na(s)+Na2O2(s)=2Na2O(s)△H1=-317kJ?mol-1
(3)已知:C(s,石墨)+O2(g)═CO2(g)△H1=-393.5kJ?mol-1;
2H2(g)+O2(g)═2H2O(l)△H2=-571.6kJ?mol-1;
2C2H2(g)+5O2(g)═4CO2(g)+2H2O(l)△H2=-2599kJ?mol-1;
写出由C(s,石墨)和H2(g)生成1mol C2H2(g)的热化学方程式
2C(s,石墨)+H2(g)=C2H2(g),△H1=226.7kJ?mol-1
2C(s,石墨)+H2(g)=C2H2(g),△H1=226.7kJ?mol-1
.