摘要:11.25℃时.在25mL0.1mol/L的氢氧化钠溶液中.逐滴加入0.2mol/LCH3COOH.溶液的pH的变化曲线如图所示.下列分析的结论正确的是 A.B点的横座标a =12.5.且有c (Na+)=c (CH3COO-) B.C点时.c (CH3COO-)>c (Na+)>c (H+)= c (OH-) C.D点时.c (CH3COO-)+ c (CH3COOH)=2c (Na+) D.对曲线上A.B间任何一点.溶液中都有: c (Na+)>c (CH3COO-)>c (OH-) >c (H+)
网址:http://m.1010jiajiao.com/timu3_id_392859[举报]
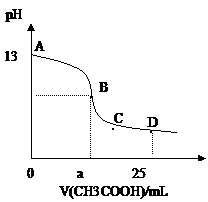 A.B点的横座标a==12.5,且有c(Na+)==c(CH3COO—)
A.B点的横座标a==12.5,且有c(Na+)==c(CH3COO—)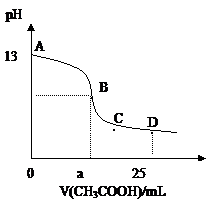 A.B点的横座标a==12.5,且有c(Na+)==c(CH3COO—)
A.B点的横座标a==12.5,且有c(Na+)==c(CH3COO—)