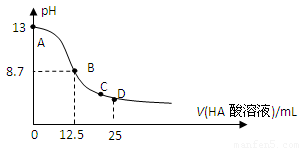
��Ŀ����
25��ʱ����25mL0.1mol/L������������Һ�У���μ���0.2mol/LCH3COOH����Һ��pH�ı仯������ͼ��ʾ�����з����Ľ�����ȷ����
 A.B������a==12.5������c(Na+)==c(CH3COO��)
A.B������a==12.5������c(Na+)==c(CH3COO��)
B.C��ʱ��c(CH3COO��)��c(Na+)��c(H+)= c(OH��)
C.D��ʱ��c(CH3COO��)+ c(CH3COOH)==2c(Na+)
D.��������A��B���κ�һ�㣬��Һ�ж��У�
c(Na+)��c(CH3COO��)��c(OH��) ��c(H+)
C
����:
A����,����߸պ÷�Ӧʱ,����������ˮ��,��Һ�ʼ���,��c(Na+)������c(CH3COO��)��B����,��c(H+)= c(OH��)ʱ,���ݵ���غ�c(Na+)==c(CH3COO��)��C��ȷ,���������غ㣻D����,��ʼ�� c(OH��) �� c(CH3COO��)

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
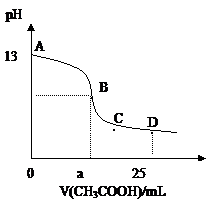
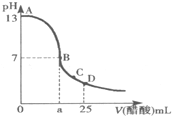 25��ʱ����25mL 0.1mol?L-1��NaOH��Һ�У���μ���0.2mol?L-1��CH3COOH��Һ����Һ��pH����������ϵ��ͼ�����з�����ȷ���ǣ�������
25��ʱ����25mL 0.1mol?L-1��NaOH��Һ�У���μ���0.2mol?L-1��CH3COOH��Һ����Һ��pH����������ϵ��ͼ�����з�����ȷ���ǣ�������