摘要:9.已知:C(s)+O2(g) = CO2(g),△H=-393.5 kJ/mol H2(g)+ O2 (g)=H2O(g),△H=-241.5 kJ/mol 欲得相同热量.需充分燃烧 C和 H2的质量比约为 ( ) A.12:3.25 B.3.25:12 C.1:1 D.6:1
网址:http://m.1010jiajiao.com/timu3_id_226306[举报]
已知:C(s)+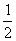 O2(g)
O2(g)  CO(g);ΔH=-110.5 kJ·mol-1?①
CO(g);ΔH=-110.5 kJ·mol-1?①
C(s)+O2(g)![]() CO2(g);ΔH=-393.51 kJ·mol-1②
CO2(g);ΔH=-393.51 kJ·mol-1②
计算下列反应的反应热ΔH:C(s)+CO2(g) ![]() 2CO(g)( )?
2CO(g)( )?
A.-283.01 kJ·mol? B.172.51 kJ·mol-1??
C.283.1 kJ·mol-1?? D.504.00 kJ·mol-1??
查看习题详情和答案>>已知:①C(s) +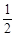 O2(g)
O2(g) CO(g);△H =" –" 110.5 kJ · mol – 1
②C(s) + O2(g) CO2(g);△H =" –" 393.51 kJ · mol – 1
则反应:C(s) + CO2(g) 2CO(g)的△H为 ( )
| A.– 283.01 kJ · mol – 1 | B.+ 172.51 kJ · mol – 1 |
| C.+ 283.01 kJ · mol – 1 | D.+ 504.00 kJ · mol – 1 |
已知:①C(s) + O2(g)
O2(g) CO(g);△H =" –" 110.5 kJ · mol – 1
②C(s) + O2(g)
CO2(g);△H ="
–" 393.51 kJ · mol – 1
则反应:C(s) + CO2(g) 2CO(g)的△H为 ( )
A.– 283.01 kJ · mol – 1 B.+ 172.51 kJ · mol – 1
C.+ 283.01 kJ · mol – 1 D.+ 504.00 kJ · mol – 1
查看习题详情和答案>>
 O2(g)
O2(g)
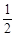 O2(g)
O2(g)