��Ŀ����
��ͼ����ʾΪijһ�ͺŶ����ܣ������˷ֱ��ΪA��B��ͼ��Ϊ���ķ����������ߣ����������ʾ�ļ����ѿ����壬Ϊȷ���ö����ܵļ��ԣ��ö��õ���ĵ��赵���в�����
��1�����ȶԶ��õ�����л�е���㣬Ȼ�졢�ڱ�����ȷ������ѡ�á�100�����в������ڲ���ǰҪ���еIJ�������Ϊ��
��2�������õ���ĺ����������ܵ�A�ˡ��ڱ���������ܵ�B������ʱ������ָ��ƫת�ǶȺܴ������ʵ����Ӻ���ָ��ƫת�ǶȺ�С��������������֪�ö����ܵ�����Ϊ
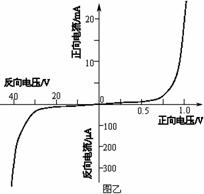
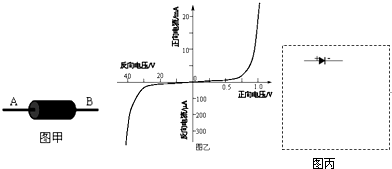
��3�������ö����ܼ��Ϸ���30V��ѹʱ���÷��������������ֵ���ṩ�������У�
��ֱ��40V��Դ��
�ڵ�����A1������0.6A������Լ10����
�۵�����A2������50��A������Լ100����
�ܵ�ѹ��V������Ϊ50V������Լ100k����
�ݻ������������ܵ���100����
���ء��������ɣ�
���ṩ�������������Ӧѡ��

��1�����ȶԶ��õ�����л�е���㣬Ȼ�졢�ڱ�����ȷ������ѡ�á�100�����в������ڲ���ǰҪ���еIJ�������Ϊ��
����ڱ��ʶ̽ӣ����ڵ�����ť��ʹָ��ָ�������0�̶ȴ���
����ڱ��ʶ̽ӣ����ڵ�����ť��ʹָ��ָ�������0�̶ȴ���
��2�������õ���ĺ����������ܵ�A�ˡ��ڱ���������ܵ�B������ʱ������ָ��ƫת�ǶȺܴ������ʵ����Ӻ���ָ��ƫת�ǶȺ�С��������������֪�ö����ܵ�����Ϊ
B
B
�ˣ��A����B������3�������ö����ܼ��Ϸ���30V��ѹʱ���÷��������������ֵ���ṩ�������У�
��ֱ��40V��Դ��
�ڵ�����A1������0.6A������Լ10����
�۵�����A2������50��A������Լ100����
�ܵ�ѹ��V������Ϊ50V������Լ100k����
�ݻ������������ܵ���100����
���ء��������ɣ�
���ṩ�������������Ӧѡ��
��
��
����ڡ��ۡ����������߿��ڻ���ʵ���·ͼ���������ܵķ����ѻ���ͼ�У�����������1������ʱ�Ƚ���ŷķ���㣬��������ڱ��ʶ̽ӣ����ڵ�����ť��ʹָ��ָ�������0�̶ȴ����ٽ��в�����
��2�������ܾ��е����ԣ����ݽ�ͨ���õ����ĵ�����С�����ж϶��йܵ���������
��3�������ܼ��Ϸ����ѹʱ������ܴ�����С�����ݶ����ܵķ������������ѹ����Ĺ�ϵ��ѡ�������������ӷ������ڶ����ܵķ������ܴ�����Ӧ�ӳɷ�ѹʽ�ӷ���
��2�������ܾ��е����ԣ����ݽ�ͨ���õ����ĵ�����С�����ж϶��йܵ���������
��3�������ܼ��Ϸ����ѹʱ������ܴ�����С�����ݶ����ܵķ������������ѹ����Ĺ�ϵ��ѡ�������������ӷ������ڶ����ܵķ������ܴ�����Ӧ�ӳɷ�ѹʽ�ӷ���
��� �⣺��1���ڲ���ǰҪ���еIJ�������Ϊ��������ڱ��ʶ̽ӣ����ڵ�����ť��ʹָ��ָ�������0�̶ȴ���
�⣺��1���ڲ���ǰҪ���еIJ�������Ϊ��������ڱ��ʶ̽ӣ����ڵ�����ť��ʹָ��ָ�������0�̶ȴ���
��2�������õ���ĺ����������ܵ�A�ˡ��ڱ���������ܵ�B������ʱ������ָ��ƫת�ǶȺܴ�ʱ����������ͨ���������ʵ����Ӻ���ָ��ƫת�ǶȺ�С�������ܷ����ֹ�����ںڱ��ʽӵ�Դ��������˵��������B��Ϊ�����ܵ�������
��3�������ܼ��Ϸ����ѹʱ������ܴ�����С���ʵ�����Ӧѡ��ۣ�
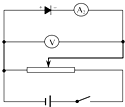
�����ܵķ������ܴ�Զ���ڵ��������裬���Ե����������ڽӷ������ڶ����ܵķ������ܴ�����Ӧ�ӳɷ�ѹʽ�ӷ�����·��ͼ��ʾ��
�ʴ�Ϊ��
��1������ڱ��ʶ̽ӣ����ڵ�����ť��ʹָ��ָ�������0�̶ȴ���
��2��B
��3���ۣ���·ͼ��ͼ��ʾ��
 �⣺��1���ڲ���ǰҪ���еIJ�������Ϊ��������ڱ��ʶ̽ӣ����ڵ�����ť��ʹָ��ָ�������0�̶ȴ���
�⣺��1���ڲ���ǰҪ���еIJ�������Ϊ��������ڱ��ʶ̽ӣ����ڵ�����ť��ʹָ��ָ�������0�̶ȴ�����2�������õ���ĺ����������ܵ�A�ˡ��ڱ���������ܵ�B������ʱ������ָ��ƫת�ǶȺܴ�ʱ����������ͨ���������ʵ����Ӻ���ָ��ƫת�ǶȺ�С�������ܷ����ֹ�����ںڱ��ʽӵ�Դ��������˵��������B��Ϊ�����ܵ�������
��3�������ܼ��Ϸ����ѹʱ������ܴ�����С���ʵ�����Ӧѡ��ۣ�
�����ܵķ������ܴ�Զ���ڵ��������裬���Ե����������ڽӷ������ڶ����ܵķ������ܴ�����Ӧ�ӳɷ�ѹʽ�ӷ�����·��ͼ��ʾ��
�ʴ�Ϊ��
��1������ڱ��ʶ̽ӣ����ڵ�����ť��ʹָ��ָ�������0�̶ȴ���
��2��B
��3���ۣ���·ͼ��ͼ��ʾ��
���������⿼������ܷ����������ߵ�ʵ�飬������Ҫע�����ñ��ĺڱ��ʽ��ڲ���Դ��������Ҫ�ܸ�����ֵ�ȽϷ�ѡ��������Ľӷ���

��ϰ��ϵ�д�
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
�����Ŀ