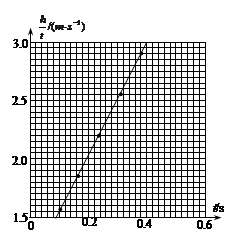��Ŀ����
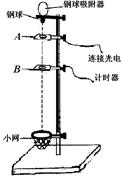
ͼ����ijʵ��С���������浼��̽��������ٶ������Ĺ�ϵ��ʵ��װ�á������ù���Ų���������ڹ���ͨ������������ʱ�䣬�Ӷ���������ʱ���ٶȡ��ڹ����̶���С���ϣ����ػ����˶�����Ŀ���Ϊd���ò�ͬ����ͨ��ϸ����ͬһ���飬ÿ�λ��鶼��ͬһλ���ɾ�ֹ�ͷš�
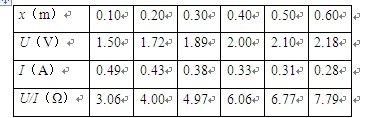
��1�����ÿ̶ȳ߲���ڹ����Ŀ���d��ͼ����ʾ����d= cm��ʵ��ʱ��С����ͼʾλ���ɾ�ֹ�ͷţ������ּ�ʱ�������ڹ���ͨ����������õ�ʱ�䦤t=2.0��10-2s�����ʱ���ٶȴ�СΪv= m/s��
��2��ʵ���пɽ�����Ϊϸ�߶Ի���������빳��������С��ȣ����������m�뻬�������M��Ӧ����Ĺ�ϵΪ ��
��3��������鹳�������m�Ͷ�Ӧ�ڹ���ͨ������ŵ�ʱ�䦤t��������ڹ���ͨ�������ʱС�����ٶ�v��ͨ�������������ͼ���о�������ٶ������Ĺ�ϵ����������ʱӦ���� ��ѡ�v-m����v2-m����ͼ��ͼ��б��k= �� ���������ٶ�g����������M�����������ż����L��ʾ����
(1 )0.52 0.26 ��2��mԶС��M (3) v2--m 2gL/M
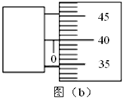
���������������1���α꿨����ʮ�ֶȵģ�����ͼ�ж���Ϊ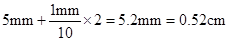 ������ͨ������ŵ��ٶ�Ϊ
������ͨ������ŵ��ٶ�Ϊ ��
��
��2����mԶС��Mʱ��ϵͳ���ٶȺ�С����ʱ��m�� ,��a��Сʱ������Ϊ
,��a��Сʱ������Ϊ ��
��
��3����������ʱӦ�跨��ͼ��ֱ�ߣ������ų���ȷ�������������㣬���� ����֪
����֪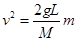 ������v2-mͼ����һ��ֱ�ߣ���б�ʵ���2gL/M��
������v2-mͼ����һ��ֱ�ߣ���б�ʵ���2gL/M��
���㣺��ʵ�飺��֤ţ���˶����ɡ�

��ƽľ�塢ϸ���ס���Ƥ���������Ƶ�������֤����ƽ���ı��η���ʵ�飬Ϊ��ʹʵ���ܹ�˳�����У��Ҿ�����С���� ����ѡ��ǰ����ĸ��
| A���ò�������ϸ����ʱ������Ӧ�ص��ɵ����ߣ�����ƽľ��ƽ�� |
| B����ϸ���ױ���ȳ� |
| C����ͬһ��ʵ���У�������ϸ����ʱ����ʹ��㵽��ͬһλ�� |
| D���ò���������ϸ����ʱ���������н�Խ��Խ�� |
��6�֣�ijͬѧ����̽���������ϳɵĹ��ɡ�ʵ��ʱ��
��1����Ҫ�����ǣ�
| A�������Ϸ�һ�鷽ľ�壬�ڷ�ľ������һ�Ű�ֽ����ͼ���Ѱ�ֽ���ڷ�ľ���ϣ� |
| B����ͼ������Ƥ����һ�˹̶��ڰ��ϵ�A�㣬����Ƥ������һ��˩������ϸ����ϸ������һ��ϵ�����ף� |
| C�����������ɲ����Ʒֱ�ס���ף����ɽǶȵ�����Ƥ����ʹ��Ƥ���쳤����㵽��ijһλ��O. ��¼��O���λ�ã������������ɳӵ�ʾ���� |
| D����ѡ�õı�ȣ���Ǧ�ʺͿ̶ȳ�������ֻ���ɳӵ�����F1��F2��ͼʾ������ƽ���ı��ζ����������F�� |
F. �Ƚ���F����F�Ĵ�С�ͷ��������Ƿ���ͬ���ó����ۡ�
����������
�٣�2�֣�����Ҫ��©�IJ��������� �� ��
�ڣ�2�֣���©�����ݷֱ��� �� ��
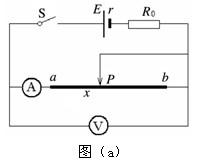
��2����2�֣���ͼ��ʾ����λͬѧ������ʵ��ʱ�õ��Ľ���������ж�����ʵ�����ȽϷ���ʵ����ʵ����______.

һ������ȤС��������װ�ò��������볤ΪL=1m�Ĺ��֮��Ļ���Ħ�������Լ�̽���������ع�������˶�ʱ���ܺ��������붯�ܱ仯�Ĺ�ϵ��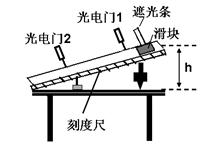
��1�� ������������֮��Ļ���Ħ��������
����ϰ�װ����������ţ����й����1��λ�ÿ��ƶ�����һ���п���Ϊd=1cm���ڹ����Ļ����Թ���ϻ���ʱ������������Ŷ������ļ�ʱ��������ʾ���ڹ���ͨ�������1��2���õ�ʱ��ֱ�Ϊ��t1����t2��ʹ������һ�����ٶ��»������ڹ���Ҷ˾�����ĸ߶ȣ�����ʹ��t1 =��t2 �������׳߲���������Ҷ˾�����ĸ߶�h="25cm" ��������֮��Ļ���Ħ������ ��=________��
��2�� ̽���������ع�������˶�ʱ���ܺ��������붯�ܱ仯�Ĺ�ϵ��
���������2������ʵ�飨1����h���䣬�ڹ���Ҷ˰�װһ�⻬�Ķ����֣���ϸ�����ӻ��������������������������������������ֱ���ɵ�����ʾ����ϸ�ߺ��������֣�����������һ�����ͼ��ʾ���������ڹ���������1����x�ɴӿ̶ȳ��϶�����ͨ���ı����ŵ�λ�ã�����ö������ݣ�ÿ�ξ��ɹ���ף��ͣ����˴���ø�Ϊͬһλ�ã������ٶ��ͷŻ��飬�����������ļ�ʱ����ʾ��ÿ���ڹ���ͨ�������1���õ�ʱ�䦤t�������ü�¼�����±���ʾ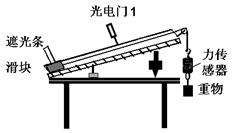
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| x(cm) | 10.0 | 22.6 | 50.0 | 62.4 |
| ��t(s) | 0.0101 | 0.0067 | 0.0045 | 0.0040 |
| V(m/s) | 0.99 | 1.50 | 2.24 | |
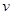 = ________����������λ��Ч���֣�
= ________����������λ��Ч���֣���Ҫ��֤�ܺ��������붯�ܱ仯�Ĺ�ϵ������Ҫ������������ǣ� ��
A����ѧ������������
B . �������ڹ���������
C�����������
D. ����ÿ�δӾ�ֹ��ʼ������������õ�ʱ��
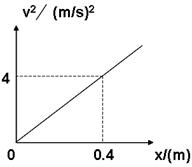
��һλͬѧÿ��ʵ��õ�������������ֵ��Ϊ19.8N������������ͼ��������������v2----xͼ����ͼ��ʾ������ͼ������������������M=________�����������ٶ�g=9.8m/s2��