��Ŀ����
�ڡ�̽�����ٶ������������Ĺ�ϵ��ʵ���У�ij��ͬѧ����ͼ1װ�ã����ÿ��Ʊ����������о�С����������������С�����ٶ��������Ĺ�ϵ��
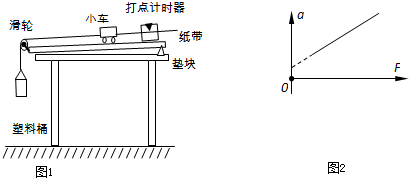
��1�����д�ʩ�в���Ҫ�Ͳ���ȷ����
A������Ҫƽ��Ħ������ʹС���ܵ���������ϸ����С��������
B��ƽ��Ħ�����ķ�����������СͰ����������ʹС�������ٻ���
C��ÿ�θı���С����������Ҫ����ƽ��Ħ����
D��ʵ����ͨ��������Ͱ�������������ı�С���ܵ�������
E��ʵ����Ӧ�ȷ�С�����ٿ�����ʱ���ĵ�Դ
��2��ij��ͬѧʵ��ó����ݣ�����a-Fͼ����ͼ2����ʵ���г��ֵ����������

��1�����д�ʩ�в���Ҫ�Ͳ���ȷ����
BCE
BCE
��A������Ҫƽ��Ħ������ʹС���ܵ���������ϸ����С��������
B��ƽ��Ħ�����ķ�����������СͰ����������ʹС�������ٻ���
C��ÿ�θı���С����������Ҫ����ƽ��Ħ����
D��ʵ����ͨ��������Ͱ�������������ı�С���ܵ�������
E��ʵ����Ӧ�ȷ�С�����ٿ�����ʱ���ĵ�Դ
��2��ij��ͬѧʵ��ó����ݣ�����a-Fͼ����ͼ2����ʵ���г��ֵ����������
��ľ����ǹ���ƽ��Ħ�������ȣ�
��ľ����ǹ���ƽ��Ħ�������ȣ�
��������1��̽�����ٶ������Ĺ�ϵʵ��ʱ����Ҫƽ��Ħ������ƽ��Ħ����ʱ��Ҫ��С���������������ƽ��Ħ����������Ҫ���κζ�����ƽ��Ħ����ʱ����������ľ�巽��ķ�������Ħ����������mgsin��=��mgcos�ȣ�����Լ��m��ֻ��Ҫƽ��һ��Ħ�����������������Ƚ�ͨ����ʱ���ĵ�Դ���ٷſ�С����С���ļ��ٶ�Ӧ���ݴ����ֽ�������
2������ͼ2���ص�ó�����F=0ʱ����ļ��ٶȲ�Ϊ0��������ţ�ٵڶ����ɽ��
2������ͼ2���ص�ó�����F=0ʱ����ļ��ٶȲ�Ϊ0��������ţ�ٵڶ����ɽ��
����⣺��1��A��ʵ������Ҫƽ��Ħ������ʹС���ܵ���������ϸ����С������������A��ȷ��
B��ƽ��Ħ�����ķ������ǣ�С����ֽ��������С��ǰ�治��СͰ����С������б���ϸ�С��һ�����ٶȣ���С���ܷ�������ֱ���˶�����B����
C������ƽ��Ħ����֮����Mgsin��=��Mgcos�ȣ���tan��=�̣���������С���������Ƿ�ı䣬С�����ܵĻ���Ħ����������С����������б��ķ�����
�ı�С���������ı���С������������Ҫ����ƽ��Ħ��������C����
D��ʵ���У�Ҫ��֤����Ͱ�������������������ԶС��С������������������ʹ������Ͱ����������������������Ƶ���ϸ����С����������
����ʵ����ͨ��������Ͱ�������������ı�С���ܵ�����������D��ȷ��
E��ʵ����Ӧ�Ƚ�ͨ��Դ����ſ�С������E����
����ѡ����Ҫ�Ͳ���ȷ�ģ���ѡ��BCE��
��2����ͼ2��֪������F=0ʱ����ļ��ٶȲ�Ϊ0������������Ϊ0��ͼ2����Ľؾ����0��
˵���������������������ļ��ٶȴ���0������ƽ��Ħ����ʱ����ľ����ǹ���ƽ��Ħ�������ȣ���
�ʴ�Ϊ����1��BCE ��2����ľ����ǹ���ƽ��Ħ�������ȣ���
B��ƽ��Ħ�����ķ������ǣ�С����ֽ��������С��ǰ�治��СͰ����С������б���ϸ�С��һ�����ٶȣ���С���ܷ�������ֱ���˶�����B����
C������ƽ��Ħ����֮����Mgsin��=��Mgcos�ȣ���tan��=�̣���������С���������Ƿ�ı䣬С�����ܵĻ���Ħ����������С����������б��ķ�����
�ı�С���������ı���С������������Ҫ����ƽ��Ħ��������C����
D��ʵ���У�Ҫ��֤����Ͱ�������������������ԶС��С������������������ʹ������Ͱ����������������������Ƶ���ϸ����С����������
����ʵ����ͨ��������Ͱ�������������ı�С���ܵ�����������D��ȷ��
E��ʵ����Ӧ�Ƚ�ͨ��Դ����ſ�С������E����
����ѡ����Ҫ�Ͳ���ȷ�ģ���ѡ��BCE��
��2����ͼ2��֪������F=0ʱ����ļ��ٶȲ�Ϊ0������������Ϊ0��ͼ2����Ľؾ����0��
˵���������������������ļ��ٶȴ���0������ƽ��Ħ����ʱ����ľ����ǹ���ƽ��Ħ�������ȣ���
�ʴ�Ϊ����1��BCE ��2����ľ����ǹ���ƽ��Ħ�������ȣ���
���������⿼����ʵ��ע�����ʵ�����ݴ���������֪��ʵ��ԭ����ע���������ȷ���⣻̽�����ٶ������������Ĺ�ϵʵ��ʱ��Ҫƽ��С���ܵ���Ħ��������ƽ��Ħ��������ƽ��Ħ�������������ƽ��Ħ������С���ܵ��ĺ��������ڹ����������

��ϰ��ϵ�д�
�����Ŀ
 ijͬѧ������̽�����ٶ������Ĺ�ϵ����ʵ���У�ʹ����ͼʾ��װ�ã���С�����ڹ���ϣ�С����һ������һ����������ʱ����ֽ������һ����ϸ���빳�����ӣ�ϸ����������֣�
ijͬѧ������̽�����ٶ������Ĺ�ϵ����ʵ���У�ʹ����ͼʾ��װ�ã���С�����ڹ���ϣ�С����һ������һ����������ʱ����ֽ������һ����ϸ���빳�����ӣ�ϸ����������֣�
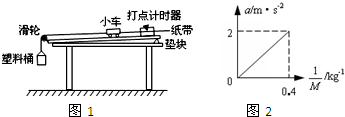

 ������̽�����ٶ�������������ϵ����ʵ��ʱ��������ͼ��ʾ��ʵ��װ�ã�������ͨ�������϶�С���ڳ�ľ�������ȼ���ֱ���˶�������С��������M��ʾ������������m��ʾ�����ٶ���a��ʾ��
������̽�����ٶ�������������ϵ����ʵ��ʱ��������ͼ��ʾ��ʵ��װ�ã�������ͨ�������϶�С���ڳ�ľ�������ȼ���ֱ���˶�������С��������M��ʾ������������m��ʾ�����ٶ���a��ʾ��