��Ŀ����
���ڸ����Ϊ����������{an}���������ai+i��i=1��2��3������Ϊ��ȫƽ�������������{an}���С�P���ʡ�����������{an}�Ƿ���С�P���ʡ������������{an}����ͬһ���е�{bn}����{bn}ͬʱ��������������������b1��b2��b3������bn��a1��a2��a3������an��һ�����У�������{bn}���С�P���ʡ����������{an}���С��任P���ʡ���
����������{an}��ǰn���Sn=
| n | 3 |
�������ж�����1��2��3��4��5������1��2��3������11�Ƿ���С��任P���ʡ������д����ʵ�������д����Ӧ������{bn}�����ߴ����ʵ�˵�����ɣ�
����������������A��1��2��3������n��ij���Ѿ���֤��n��[12��m2]��m��5��ʱ������A���С��任P���ʡ�����֤������n��[m2+1����m+1��2]ʱ������AҲ���С��任P���ʡ���
����������������֪an=n2-n��n��N*��������ai+i=i2��i=1��2��3��������ȫƽ����������{an}���С�P���ʡ���
��������������֪������1��2��3��4��5���С��任P���ʡ�������{bn}Ϊ3��2��1��5��4������1��2��3����11�����С��任P���ʡ���������1��2��3����11�����С��任P���ʡ���
������n=m2+j��1��j��2m+1����h=4m+4-j-1����h��[12��m2]���ɴ˿�֪��n��[m2+1����m+1��2]ʱ������AҲ���С��任P���ʡ���
��������������֪������1��2��3��4��5���С��任P���ʡ�������{bn}Ϊ3��2��1��5��4������1��2��3����11�����С��任P���ʡ���������1��2��3����11�����С��任P���ʡ���
������n=m2+j��1��j��2m+1����h=4m+4-j-1����h��[12��m2]���ɴ˿�֪��n��[m2+1����m+1��2]ʱ������AҲ���С��任P���ʡ���
����⣺����n��2ʱ��an=Sn-Sn-1��1�֣�=
(n2-1)-
[(n-1)2-1]=n2-n����2�֣�
��a1=0������an=n2-n��n��N*������3�֣�
����ai+i=i2��i=1��2��3��������ȫƽ����������{an}���С�P���ʡ�����4�֣�
��������1��2��3��4��5���С��任P���ʡ�����5�֣�
����{bn}Ϊ3��2��1��5��4����6�֣�
����1��2��3����11�����С��任P���ʡ�����7�֣�
��Ϊ11��4��ֻ����5�ĺͲ��ܹ�����ȫƽ������
��������1��2��3����11�����С��任P���ʡ�����8�֣�
������n=m2+j��1��j��2m+1��
ע���m+2��2-��m2+j��=4m+4-j��
��h=4m+4-j-1��
����1��j��2m+1��m��5������h=4m+4-j-1��2m+2��12��
��m2-h=m2-4m-4+j+1��m2-4m-2��m2-4m-2=��m-2��2-6��0��
����h��m2��
��h��[12��m2]����10�֣�
��Ϊ��n��[12��m2]��m��5��ʱ������{an}���С��任P���ʡ���
����1��2����4m+4-j-1�������г�a1��a2��a3����ah��ʹ��ai+i��i=1��2����h������ƽ��������11�֣�
���⣬4m+4-j��4m+4-j+1����m2+j�����෴˳�����У�������Ϊm2+j����4m+4-j+1��4m+4-j��
ʹ�ã�4m+4-j��+��m2+j��=��m+2��2����4m+4-j+1��+��m2+j-1��=��m+2��2������12�֣�
����1��2����4m+4-j-1��4m+4-j����m2-1+j��m2+j
�����ų�a1��a2��a3����ah��m2+j����4m+4-j����ai+i��i=1��2����m2+j������ƽ��������13�֣�
| n |
| 3 |
| n-1 |
| 3 |
��a1=0������an=n2-n��n��N*������3�֣�
����ai+i=i2��i=1��2��3��������ȫƽ����������{an}���С�P���ʡ�����4�֣�
��������1��2��3��4��5���С��任P���ʡ�����5�֣�
����{bn}Ϊ3��2��1��5��4����6�֣�
����1��2��3����11�����С��任P���ʡ�����7�֣�
��Ϊ11��4��ֻ����5�ĺͲ��ܹ�����ȫƽ������
��������1��2��3����11�����С��任P���ʡ�����8�֣�
������n=m2+j��1��j��2m+1��
ע���m+2��2-��m2+j��=4m+4-j��
��h=4m+4-j-1��
����1��j��2m+1��m��5������h=4m+4-j-1��2m+2��12��
��m2-h=m2-4m-4+j+1��m2-4m-2��m2-4m-2=��m-2��2-6��0��
����h��m2��
��h��[12��m2]����10�֣�
��Ϊ��n��[12��m2]��m��5��ʱ������{an}���С��任P���ʡ���
����1��2����4m+4-j-1�������г�a1��a2��a3����ah��ʹ��ai+i��i=1��2����h������ƽ��������11�֣�
���⣬4m+4-j��4m+4-j+1����m2+j�����෴˳�����У�������Ϊm2+j����4m+4-j+1��4m+4-j��
ʹ�ã�4m+4-j��+��m2+j��=��m+2��2����4m+4-j+1��+��m2+j-1��=��m+2��2������12�֣�
����1��2����4m+4-j-1��4m+4-j����m2-1+j��m2+j
�����ų�a1��a2��a3����ah��m2+j����4m+4-j����ai+i��i=1��2����m2+j������ƽ��������13�֣�
���������⿼�����е����ʺ�Ӧ�ã�����ʱҪע������ܽ�������������

��ϰ��ϵ�д�
�����Ŀ
 �����
����� (
( =1��2��3�� )Ϊ��ȫƽ�������������
=1��2��3�� )Ϊ��ȫƽ�������������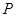 ���ʡ�����������
���ʡ����������� ��
�� ��
�� ��һ�����У�
��һ�����У� ���
��� ��
�� ��1��2��3��4��5��
��1��2��3��4��5�� ��1��2��3��4��5��6��7��8��9��10��11.
��1��2��3��4��5��6��7��8��9��10��11. �����
����� ��
�� =1��2��3������Ϊ��ȫƽ�����������
=1��2��3������Ϊ��ȫƽ�����������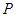 ���ʡ�����������
���ʡ����������� ����
���� ��
�� ��һ�����У�������
��һ�����У������� ���
��� ��������1��2��3��4��5����1��2��3������11������
��������1��2��3��4��5����1��2��3������11������ �����
����� (
( =1��2��3����)Ϊ��ȫƽ�����������
=1��2��3����)Ϊ��ȫƽ����������� ���ʡ���
���ʡ��� ����
���� ��
�� ��һ�����У�������
��һ�����У������� ���
��� ��֤������
��֤������ ��1��2��3������
��1��2��3������ ʱ��
ʱ�� ʱ������
ʱ������