��Ŀ����
14����������¼Ƭ����ϵ��й�����ȫ���ע��ʳ���ȳ������ж�ν��������ò�ͬ����ķ���������������ζʳƷ��������ƣ���ף����飬�ݲ˵ȣ���������ش��������⣺��1���ú������������ƵĹ����У����ѾƳ��ֻ�ɫ��ԭ���Ǻ�ɫ����Ƥ�е�ɫ���ܽ��ڷ���Һ�����Ѿ������ܷ⣬���ڴ�žͻ���ᣬ���Ż�����������ʺ��¶�Ӧ����18-25��
��2���ڸ�������������У���������������˶����ķ��ͣ���������Ҫ���õ���ëù�����������ø�ܽ������еĵ����ʷֽ��С���ӵ��ĺͰ�����
��3�������ݲ˹������������εĺ����仯�������Ӻ����
��4����������ʳ�Ĺ�����Ҳ�������ֽܷ���ά�ص�����ɲ��øչ�Ⱦ��ɫ������ɸѡ�������Ʒ��ϸ��Ũ�Ƚϵͣ�����ͨ��ѡ��������������ά�طֽ����Ũ�ȣ�Ϊ�˴�����ά�طֽ����ijͬѧ����ƽ�廮�߷����н��֣������ڶ����Լ����Ļ��߲���ʱ��Ӧ������һ�λ��ߵ�ĩ�˿�ʼ����
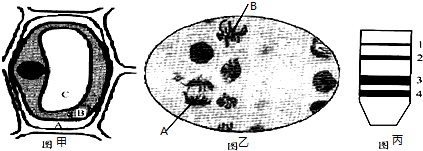
��5����������ķ������д��������أ�������ͨ���ֽ����ص�ϸ�����佫�⣬�������з�����ֽ����ص�ϸ������������Ҫ����A��
�ټ����آڲ������آۼ���֬�� �ܲ�����֬�Ǣݼ������Ǣ��������Ǣ�KH2PO4��Na2HPO4��MgSO4•7H2O���KH2PO4��Na2HPO4��MgSO4•7H2O
A���٢ۢݢ�B���٢ۢݢ�C���ڢܢޢ�D���٢ܢޢ�
���� 1��������������������ǽ�ĸ�������³´�л����Ϊ�������������ͣ�����������ԭ����
��1�������������£���Ӧʽ���£�C6H12O6+6H2O+6O2$\stackrel{ø}{��}$6CO2+12H2O+������
��2�������������£���Ӧʽ���£�C6H12O6$\stackrel{ø}{��}$2CO2+2C2H5OH+������
2��������������������Ǵ���������³´�л���������������ͣ�����������ԭ����
����������Դ������ʱ�������������֭�еĹ��Ƿֽ�ɴ��ᣮ
��ȱ����Դʱ����������Ҵ���Ϊ��ȩ���ٽ���ȩ��Ϊ���ᣮ
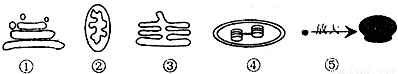
3�����븯��������������Ҫ��ëù�����³´�л���������������ͣ�
��� �⣺��1���ú������������ƵĹ����У����ѾƳ��ֺ�ɫ��ԭ���Ǻ�ɫ����Ƥ�е�ɫ���ܽ��ڷ���Һ�У����Ѿƴ�Ż�������������¶�Ӧ��Ϊ18-25�森
��2�����븯��������������Ҫ��ëù������������ԭ���ǣ�����ø�ܽ������еĵ����ʷֽ��С���ӵ��ĺͰ����֬��ø�ɽ�֬��ˮ��Ϊ���ͺ�֬���ᣮ
��3���ݲ����������У��ڳ����������κ����ܵͣ������ỹԭ���ķ�ֳ���ٽ������λ�ԭΪ�������Σ����������������������ߣ���������ʱ���ӳ��������������ֳ���������ᣬ�����������λ�ԭ���ķ�ֳ���������εĺ������ͣ�
��4��ɸѡ�ܹ�������ά�ص�ϸ�����ɲ��øչ���Ⱦɫ���������Ʒ��ϸ��Ũ�Ƚϵͣ�����ͨ��ѡ��������������ά�طֽ����Ũ�ȣ�ijͬѧ����ƽ�廮�߷����н��֣������ڶ����Լ����Ļ��߲���ʱ��Ӧ�ô���һ�λ��ߵ�ĩ�˿�ʼ���ߣ�
��5���ֽ����ص�ϸ����������������������Ӧ�ü����л�̼Դ���������ǣ�����ֽ����صľ����������ӹ����Ϸ�����ѡ����������Ӧ��������ΪΨһ��Դ�������������Ϸ����ڹ��������������Ӧ�������̼���֬�ǣ�ͬʱ����Ҫ����KH2PO4��Na2HPO4��MgSO4•7H2O����ѡ��A��
�ʴ�Ϊ��
��1����ɫ����Ƥ�е�ɫ���ܽ��ڷ���Һ�� 18-25��
��2���ĺͰ�����
��3�������Ӻ����
��4��ѡ�� ĩ��
��5��A
���� ���⿼����ƺ���������������������ݲ˵�������֪ʶ��Ҫ����ʶ�Dz�����ơ����ס�������ݲ����������P���л���ͣ����չ��ơ����ס�������ݲ�������ԭ���������������������εIJⶨ�������ܽ����ѧ��֪ʶȷ���⣮

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�| ��� | �� | �� | �� | �� |
| ���� | ��Ȼ ״̬ | ������������Ũ�ȵ������ش�����ͷ�� | ����ǰ����ֽ����������������Ũ�ȵ������ش�����ͷ��Ȼ��������ֽ���� | ����ǰ����ֽ����������������Ũ�ȵ���ˮ���ش�����ͷ��Ȼ�������ϴ��� |
| ע���ƹ��Ǵ����컨ֲ�� | ||||
| A�� | ���� | B�� | ����ͱ��� | C�� | ���� | D�� | ����Ͷ��� |
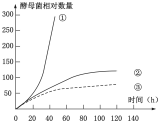 ��ͼΪ�ڵ��ݻ������У��ò�ͬ����������ĸ��ʱ������Ⱥ���������ߣ����������ֱ�Ϊ������������Һ������������Һ����ʱ����pHʹ���Ⱥ㶨�����ˣ�ÿ3h���ڸ�������Һ����Ӧ���������ǣ�������
��ͼΪ�ڵ��ݻ������У��ò�ͬ����������ĸ��ʱ������Ⱥ���������ߣ����������ֱ�Ϊ������������Һ������������Һ����ʱ����pHʹ���Ⱥ㶨�����ˣ�ÿ3h���ڸ�������Һ����Ӧ���������ǣ�������| A�� | �٢ڢ� | B�� | �ۢڢ� | C�� | �ۢ٢� | D�� | �ڢ٢� |
| A�� | �����Ǻ��Ƿ��Ӿ��л�ԭ�� | B�� | ����ֲ��ϸ�����ǵ�������ȫ��ͬ | ||
| C�� | ��ά�ء����ۡ���ԭ�ĵ��岻ͬ | D�� | ������ϸ������Ҫ�Ĵ������� |
| A�� | ���ϸ������ҪԪ���к��������ǵ� | |
| B�� | ����֬����DNA�Ȼ���������Ԫ�أ������������Ĵ���Ԫ�� | |
| C�� | ������ϸ���к������Ļ�������ˮ | |
| D�� | ���˵�һ���У�ϸ���е�����ˮ/���ˮ�ı�ֵ������ |