��Ŀ����
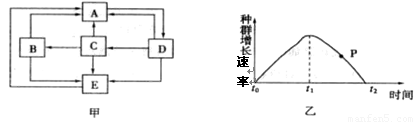
ͼ������I����ֱ��ʾ����A��������������ø����������������P����������仯���̡��������������ȷ���� ?
 ?
?
- A.ad�α�ʾ�����������£�����A��������P��Ҫ�Ļ��
- B.����ø����Ϊ���������÷�Ӧ����b�������Ͻ������ƶ�
- C.�������ӷ�Ӧ��A��������ͼ�����ߵ�ԭ����״�������ı�
- D.�����ߢ�Ϊ����ø�������µ����ߣ��ı�ø����������b�������Ͻ������ƶ�
���������
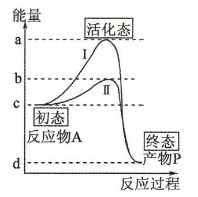
��ܶ��壺���Ӵӳ�̬ת��Ϊ��������ѧ��Ӧ�Ļ�Ծ״̬����Ҫ��������Ϊ��ܣ���ø�ܽ��ͻ�ѧ��Ӧ�Ļ�ܡ�
A. ac�α�ʾ�����������£�����A��������P��Ҫ�Ļ�� ������
B.����ø����Ϊ���������÷�Ӧ����b�������Ͻ����ϡ��ƶ���λ��ab֮�䣻����
C.�������ӷ�Ӧ��A��������ͼ�����ߵ�ԭ����״�������ı䣻����
D.�����ߢ�Ϊ����ø�������µ����ߣ��ı�ø��������ø���Խ��ͣ�����Ҫ����Ļ�ܣ�b�������Ͻ������ƶ�����ȷ��
���㣺ø������������������ѧ��Ӧ������ͼ�Ƚϡ�
������������ƽ�Ϊ�������ѧ������������������������������ʵ��

I����5�֣���������ͬ�Ĵ���������ľ��ֳ�A��B��C���飬�ֱ������ͬ����ȫ����Һ�У���������������Ƕ�K+��������ͬ����ˮ��������Ҳ������ͬ�������������ֱ�������´������ش��������⣻
| ֲ�� | A | B | C |
| ���� | ���Ӹ�Ũ�ȵ�KHNO3��Һ | ע�䡰������ϼ��� | ��ȥ����ҶƬ |
��2��Bֲ��Կ������ӵ����������ܵ�����Ӱ�죬��ԭ���� ��
��3��Aֲ����������ϸ�����ܻᷢ�� ������ˮ�ֺͿ�������������һ�� �Ĺ��̡�
��4������������¸����������Ƥϸ���ܹ����е�������� �������ţ�
��ϸ����ֳ ������ˮ �۹������ ��ϸ����DNAֻת¼������
����mRNA���˿���ϸ������
II.��19�֣�����ļס�����ͼ��ӳ�˶�����̼��������ǿ�ȶ���ɫֲ�������õ�Ӱ�죬�����ͼ�ش�
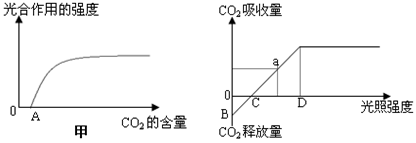
��1����������ũ������ʩũ�ҷʿ�����߹��������ʣ���ԭ��������ͼ�е� ͼ����Ҫ�ö���������Ϊ�̷ʣ������������������� Ԫ�صĺ�����
��2��������ͼ������������ƽ�������������Ҫ�� ���������������ͼ��ʾijֲ���ں���30��ʱ��������ڹ���ǿ�ȵĹ�ϵ�����¶ȵ��ڵ�25�棬������B�㽫�� �ƶ���C�㽫�� �ƶ���
��3����ͼ�е�D��ʱ�̣�С��ҶƬά������ϸ�����ܲ���ATP��ϸ������
��4����ͼ��a���c����ȣ�C3���� �����࣬���ٻ䣩
��5����12�֣�ij��ѧ������Ϊ̽����������������ѹ���ǿ�ȣ�����������ʵ�飺����ȡ��������״����ͬ��������ֲ�꣬ƽ����Ϊ7�飬�ֱ�����ܱյIJ��������С�ʵ�鿪ʼʱ�ⶨCO2��Ũ�ȣ�12Сʱ���ٴβⶨCO2��Ũ�ȡ�ʵ�������±���������ش�
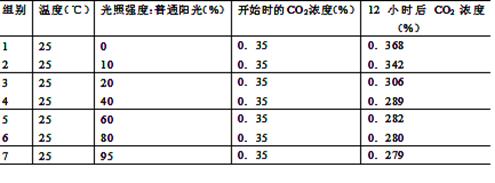
����һʵ����Ա����� ��д����ʵ����Ƶ�һ���ر����� ��
��ʵ���е�1����12Сʱ��CO2Ũ�ȱ仯��ԭ���� ��
�������ʵ�������ʹ���˲�ͬƷ�ֵ�������ֲ�꣬�������Υ���˿�ѧʵ��� ԭ��
��������7��ֲ��ͻȻ������4��������£���ʱ���ڹ��ϸ���е�ATP������ ��C5������ĺ����� ��
�ݸ�ʵ������в���ȷ����������������ѹ���ǿ�ȣ����������һ��̽����ʵ�����˼·�� ��
������ij�о���ѧϰС���ý���������״����ͬ�����������������ֻ��������ʵ�飨���±������Т�~�۴���Ҫ������ɣ������У�ʵ��I��̽���ѳ��ܷ��ڼ���A���ܴٽ��ӹ��ķ�����ʵ��II��̽�������ܷ��ڼ���B���ܴٽ��ѳ����ӹ��ķ�������ش�����������⣺
ʵ��I
| ������� | ����/�������� | һ��ʱ����ӹ�ƽ��������mg�� |
| 1 | �������� | 218 |
| 2 | �г��ѳ� | 90 |
| 3 | �� | 217 |
| ������� | ����/�������� | һ��ʱ����ѳ�ƽ��������mg�� | һ��ʱ����ӹ�ƽ��������mg�� |
| 4 | �������� | 68 | 216 |
| 5 | �� | 36 | 93 |
| 6 | �г����壬ͬʱ����ע�伤��A | 37 | 219 |
| 7 | �� | 66 | 218 |
��2��������ϱ��еġ���~�ۡ��Ĵ���������
��__________________________________________��
��_________________________________________________��
��__________________________________________________________________��
��3��ʵ���У�����A�ͼ���B�ֱ��������_______________��_______________��д���ƣ���
��4����ʵ��I�Ľ���ɵó��Ľ����ǣ�����A��______________________________�Ĺ��ܡ�
��5�����͵�5���ʵ����__________________________________ ��
��6������2�������ڵ�һ�������г��ѳ��ӵ�������ÿ��ע����������A�������Ҳ�����ͼ�м�����ɼ���A�ͼ���B��Ѫ���е�Ũ�ȱ仯���ߡ�
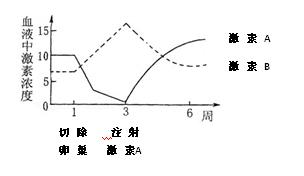
�ش�����I����������
I(16��)�±���һλ���ߵ�ѪҺ�������鱨�浥�еIJ��ּ�������ݴ˻ش��������⣺
|
��Ŀ���� |
��д |
��� |
��λ |
�ο���Χ |
|
�ȱ�ת��ø |
ALT |
67 �� |
IU/L |
0-40 |
|
���� |
BUN |
4.34 |
Mmol/L |
2.5-7 |
|
֬���� |
HDL-C |
0.19 �� |
mmol/L |
1.16-1.55 |
|
������ |
GLU |
8.65 �� |
mmol/L |
3.9-6.1 |
(1)�ȱ�ת��ø��������ϸ���ڵ�����������________________��
(2)�û��߶�οո���Ѫ��죬��ѪҺ�������Ǻ�����Ϊ����8.65���ң���ô���Գ����ж��仼���IJ����������______________������ģ��Ʋ�û���Ѫ֬����___________(��ƫ��ƫ��)
(3)�������û���ϲ�ø�֬��ʳ��ҽ������Ӧ���һЩ��__________�϶��ʳ�
(4)��������������谱���ᣬ���Ƿ�Ҳ��С��ı��谱������?ij������ȤС��ʹ����������̽����
ʵ��ԭ�������谱�����Ƕ����岻�ܺϳɡ�ֻ�ܴ�ʳ���л�õİ����ᡣ������ȱ�����谱����ʱ���ͻ�ֱ��Ӱ��_________������Ӫ���������������ӻ�����
��ѡ�������þߣ�20ֻ��������״����ͬ����������С��װ�ڲ�ͬ�Լ�ƿ�е�20�ְ����ᡢ���������ʺͰ������ʳ���ƽ�ȡ�
ʵ�鲽�裺
��һ����ʳ��ȡһ�����IJ��������ʺͰ������ʳ����뺬���ͱ������˵�20�ְ����ᣬ���Ƴ�ʳ��A��
ȡ�����IJ��������ʺͰ������ʳ�����_______________________�����Ƴ�ʳ��B��
�ڶ������飺��20ֻ����ƽ���ֳɼ������飬�ֱ��������¼�����ء�
��������ι������ÿ����ι������ʳ��A:_______________________________�����������������������ͬ��
ʵ����Ԥ�⼰���ۣ�
|
ʵ���� |
ʵ����� |
|
_________________________________________ |
�������Ǵ���ı��谱���� |
II(10��)��30ͼ��ʾ�ļ�������һ��ҹ�ڶ�CO2�����պ��ͷ��������ش�
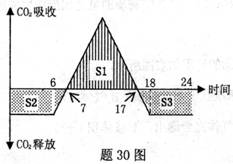
(1)����_________ʱ�л���Ļ���������ࡣ
(2)����ͼ��S1��S2��S3���ߵĹ�ϵΪ__________(��ѧ����ʽ)ʱ�������ײ�������������
(3)�����ں����ڼ�ᷢ���ø�����ԭ����_____________
(4)���Ǵ����֡����ݡ������´��������Ҫ����������______________________��
(5)ͼΪ����ȫ��ҶƬ��25��ʱ������ǿ���������ͷ��ٶȵĹ�ϵʾ��ͼ������ֲ���ҶƬ����һ�룬����ͼ�л���ֲ��ʣ��ҶƬ����ǿ���������ͷ��ٶȵĹ�ϵ����(����ÿ��ҶƬ������״̬������ͬ)��

��12�֣���������е���Ϣ�ش����⡣
I����3�֣�Ϊ̽������Һ�н�ĸ����Ⱥ�����Ķ�̬�仯��ij��ȤС�鰴�±�������й�ʵ�鲢���ڶ�����Һ�еĽ�ĸ�����м��������ݶ�μ�����ƽ��ֵ���Ƴ���ĸ��ϸ����Ŀ�仯����ͼ��������ͼ������Ϊ��ĸ��ϸ����������ش�
|
�Թܱ�� |
����Һ��mL |
��ĸ����mL |
�����¶ȣ��棩 |
|
A1��A2��A3 |
10 |
0.1 |
20 |
|
B1��B2��B3 |
10 |
0.1 |
10 |
|
C1��C2��C3 |
10 |
0.1 |
5 |
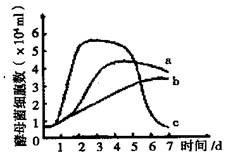
��С��̽���Ŀ����� ����ʵ������д��ڵIJ���֮���� ��ͼ������ܴ���A���ĸ����Ⱥ�����仯�������� ��
II����9�֣��±��Dz�ͬŨ�ȵ��Ͳ�������ˮ��Һ���۲���������Ӱ���ʵ����
|
��� |
��ˮ |
Ũ��a |
Ũ��b |
Ũ��c |
Ũ��d |
Ũ��e |
|
ƽ����ߣ�cm�� |
16 |
20 |
38 |
51 |
42 |
24 |
��1����ʵ�����ܷ�˵���Ͳ����������۲��������������ԣ� �������� ��
��2�������ƽ�һ��̽���Ͳ��������ٽ��۲�����������Ũ�ȷ�Χ��ʵ�鲽�裺
�� ��
��ȡͬһ������ʹ���ȷ�������ѡȡ��ߡ�������ͬ���۲����� ��
�۷ֱ� ������Ӧ����۲����磻
������ͬ�����˵�����������һ��ʱ��� ���ټ���ƽ��ֵ��