��Ŀ����
����������ض�ˮ�����������õ�ʾ��ͼ�����ʵ����֤���������õ������ԡ�
��1��ʵ����Ϻ��þߣ�ˮ���������ɣ���С��ͬ��װ�е�����ȫ����Һ�IJ���ƿ���ɣ���ͬŨ�ȵ���������Һ�����ߵȡ�
��2��ʵ�鲽�裺
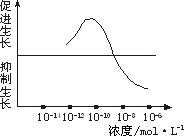
��һ��������Ũ��Ϊ10��12 mol��L��10һ10 mol��L��10��8 mol��L��10��6 mol��L����������Һ����ȡ2 ml�ֱ����A��B��C��D�ĸ�ʢ����ͬӪ��Һ������ƿ�У�E����ƿ�г�����ͬ��Ӫ��Һ�⣬�ټ���________�������ա�
�ڶ�����ȡ����________��ˮ�����磬��������¼ˮ��������ij�ʼ���ȣ��ֱ������ڵ�A��B��C��D��E��ֻ����ƿ�С�
����������A��B��C��D��E����ˮ���������________�������£�����һ��ʱ�����________�������������ˮ�������������________�����μ�¼Ϊa��b��c��d��e��
��3��ʵ����ӦΪ��________��
�𰸣�
������
������
|
������2����һ��������������ˮ���ڶ��������ơ���С��ͬ���ĵ�����������������ͬ�����ˡ�����ˮ��������ij��ȡ�ƽ��ֵ ������3��b��a��e��c��d |

��ϰ��ϵ�д�
�����Ŀ


 ��2��ʵ�鲽�裺
��2��ʵ�鲽�裺