��Ŀ����
����Ŀ�������������������ķ�ζС�ԣ������ζ���ء�Ӫ���ḻ�����������ɿڸ����dz���������������ԭ�ϡ��䲿��Ԫ�غ�����Ӫ���ɷ����¡�
Ԫ�� | ���� | Ԫ�� | ���� |
B | 6��79 | Mg | 333��73 |
Ca | 151��40 | Mn | 202��27 |
Cu | 4��18 | Na | 1650 |
Fe | 494��87 | P | 267��4 |
K | 963��87 | Zn | 19��83 |
��ش�
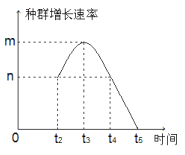
��1�����е�Ԫ�����ڴ���Ԫ�ص���________���ɿڸ����dz����Ԫ���к�����ߵ���________����Ԫ��������________���ף��ٳ�һ�����ɣ�����Ҫ��֣��õ��Ĺ�����________��
��2������ѪҺ��________���Ӻ����Ϳ��ܻᵼ�³鴤�����������Ի����֢״��
��3�����ⶨ����ɿɿڸ����dz浰���ʵİ����Ṳ��18�֣��������ڵĵ�����ȴ�г�ǧ�����֣�ԭ����________��
��4��������Ӫ����ֵ�ߵĽ���ʳƷ����Ҫ������________������д�����㣩��
���𰸡�Na��P��K��Ca��Mg Fe Ѫ�쵰�� �������� �� ���ڰ���������ࡢ��Ŀ������˳���Լ������ʵĿռ�ṹ�IJ�ͬ���µ� �������к��н϶����Ԫ�أ��ܹ�ά���������������������������ߣ�����Ѫ�쵰�����Ԫ�أ�������Ԥ��ȱ����ƶѪ��п����ɶ��ָ�ø��Ԫ�أ�����ά��ϸ�����������������Ҫ���ã��������к����������Ԫ�أ����иƵĺ����ϸߣ������ڴٽ������������ͷ���
��������
1.������Ҫ�����ӵ���ʽ���ڣ���Щ������ϸ����ijЩ���ӻ��������ɳɷ֣�������Ѫ�쵰����ɳɷ֣�þ��Ҷ���ص���ɳɷ֣����Ǽ�״�ټ��ص�ԭ�ϵȣ����ε������ܹ�ά���������������ѪҺ��Ca2+��������ʱ����鴤��������ά��ϸ����������ѹ��pHֵ����̼���������������ȡ�
2.���ϸ���Ļ�ѧԪ���У���Ϊ����Ԫ�غ���Ԫ�أ�����Ԫ�ذ���C��H��O��N��P��S��K��Ca��Mg�ȣ���Ԫ�ذ���Fe��Mn��B��Zn��Mo��Cu�ȡ�
3.���������֪���ɿڸ����dz��Ԫ����Na��P��K��Ca��Mg���ڴ���Ԫ�أ�����Na�ĺ�����ߣ����Fe��Mn��B��Zn��Cu������Ԫ�أ���Ԫ���к���������Fe��
��1���������Ϸ�����֪�����е�Ԫ�����ڴ���Ԫ�ص���Na��P��K��Ca��Mg���ɿڸ����dz����Ԫ�ذ���Fe��Mn��B��Zn��Cu�����к�����ߵ���Fe��Fe��Ѫ�쵰�����Ԫ�أ�Ѫ�쵰�������������Ĺ��ܡ�
��2�������������εĹ��ܷ�����֪������ѪҺ��Ca2+��������ʱ����鴤����
��3�����������ɰ����ᾭ����ˮ�����γɵģ����ڰ���������ࡢ����������˳���Լ��ռ�ṹ�IJ�ͬ�����µ����ʵĽṹ���ж����ԡ�
��4�����ݱ�����Ϣ��֪�������к��ж�����Ԫ�أ���Ԫ����ά������������������Ԫ�أ��纬����ߵ���Fe��Fe��Ѫ�쵰�����Ԫ�أ�����Ԥ��ȱ����ƶѪ��п����ɶ��ָ�ø��Ԫ�أ�����ά��ϸ�����������������Ҫ���ã��������к��д�����CaԪ�أ����Դٽ������������ͷ�����