��Ŀ����
����Ŀ��ij��������ƻ��������֭�����ƺ��Ĵ��¹�����������ͼ��������ش�
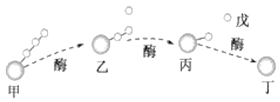
ƻ��![]() ��֭
��֭![]() ����
����![]() ����
����
��1����ƻ���ӹ��ɹ�֭�Ĺ����У���Ҫ�������ø������ø���������ֽ�ø��������ø��_________������ø�ܹ��ֽ����Ϊ______________��ʹ���ǵĹ�֭��ó��塣
��2��ƻ�������ɹ�֭���Ͳ�����,�ڴ˹����У������¶�Ҫ������______����֭���ͺ��Ƿ��оƾ�����������______________�����飬���Լ���ƾ���Ӧ����________ɫ��
��3��ƻ������____________�������£�������һ�����Ϳ��γ�ƻ���ף��ڴ˹����У�Ҫ��ʱ��Һ��ͨ�������ԭ����___________________��
��4����������ʱ���������___________����Դ����ʱ�����Ƿֽ�ɴ��ᣬ����Դ������ʱ��Ҳ��������__________���ɴ��ᡣ�����ƹ��ķ�ӦʽΪ______________________��
���𰸡���۰�����ȩ��ø �����Եİ�����ȩ�� 18��25�� ���������µ��ظ���� ���� ����� ������Ǻ���ϸ������������Ҫ���� ���� �ƾ� C2H2OH![]() CH3COOH+H2O
CH3COOH+H2O
��������
������������������ǽ�ĸ�������³´�л����Ϊ��������������������������ԭ������1�������������£���Ӧʽ���£�C6H12O6+6H2O+6O2![]() 6CO2+12H2O+��������2�������������£���Ӧʽ���£�C6H12O6
6CO2+12H2O+��������2�������������£���Ӧʽ���£�C6H12O6![]() 2CO2+2C2H5OH+������������������������Ǵ���������³´�л����������������������������ԭ��������������Դ������ʱ�������������֭�еĹ��Ƿֽ�ɴ�������ȱ����Դʱ����������Ҵ���Ϊ��ȩ���ٽ���ȩ��Ϊ������
2CO2+2C2H5OH+������������������������Ǵ���������³´�л����������������������������ԭ��������������Դ������ʱ�������������֭�еĹ��Ƿֽ�ɴ�������ȱ����Դʱ����������Ҵ���Ϊ��ȩ���ٽ���ȩ��Ϊ������
��1������ø���������ֽ�ø��������ø�Ͷ�۰�����ȩ��ø������ø�ܹ��ֽ����Ϊ�����Եİ�����ȩ�ᣬʹ���ǵĹ�֭��ó��塣
��2�����Ʒ��͵�ԭ���ǽ�ĸ����������������Ҫ�������¶���18��25�棻�����ľƾ����������������µ��ظ���ؽ��м�⣬���Լ���ƾ���Ӧ���ֻ���ɫ��
��3��ƻ�����ڴ�����������£�������һ�����Ϳ��γ�ƻ���ף��ڴ˹����У�Ҫ��ʱ��Һ��ͨ�������ԭ���Ǵ�����Ǻ���������������Ҫ������
��4�����������������Դ����ʱ�����Ƿֽ�ɴ����ȱ����Դʱ����������Ҵ���Ϊ��ȩ���ٽ���ȩ��Ϊ���ᡣ�����ƹ��ķ�ӦʽΪ![]() ��
��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�