��Ŀ����
���������У��е����ڹ��ɵ����ʵİ����ᣬ�еIJ��ǡ��������й��ɵ����ʵİ��������ϳɶ��ģ������к��еİ������Ȼ����ļ�����Ŀ�����ǣ� ��
��NH2��CH2��COOH ��NH2��CH2��CH2OH �� NH2��CH��CH2��COOH
COOH
�� NH2��CH��CH2��COOH �� NH2��CH��(CH2)4��NH2
NH2 COOH
A��2��2��2 B��3��3��2 C��4��3��3 D��3��4��3
���𰸡�
A
����������

��ϰ��ϵ�д�
�����Ŀ
��10�֣����������У��е����ڹ��ɵ����ʵİ����ᣬ�еIJ��ǡ��������еİ��������ϳ�һ�����ģ���ش��������⣺

|

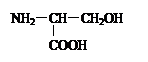
�� ��
|
��
��1���������γɷ�ʽ�� ���ں� ���ļ�����ṹʽΪ ���û������___________�ġ�
��2����ɸ��������İ�������� ��һͨʽͳһ������
��3�����������л��� �������� ���Ȼ���
��4������������Է�������������䰱�������Է���������ȼ����� ��
��5������������R������ �֣��ܵ�R���� ��

