��Ŀ����
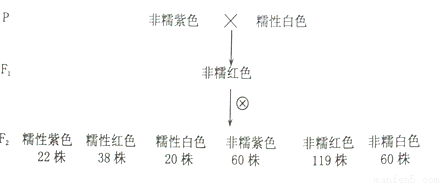
�����������Ļ�ѧԪ�أ���Ǻ����ݵ��γ��Լ�����ܵ��йء�Ѫ�Ƶ�ƽ�����״���٣������ڼ�״���ϣ����ڵļ�״���ټ��غͼ�״�ٷ��ڵĽ������йأ�������ڹ�������ͼ��ʾ����ش��������⣺

��1���������������������ϸ��ʱ������ϸ��Ĥ�ϵ�_________________���ϣ�������Һ�и�Ũ�ȵ�_____________����������ɹ�ϸ����ϣ�________________������ʹ��Ѫ��Ũ�Ƚ��͡�
��2����״���ټ��ؿ���ʹ����С������������__________���������õ����ʻ���________�������Դٽ�����ԸƵ�______������ʹ��Ѫ��Ũ�����ߡ�
��3���������ѪҺ�и����Ӻ���̫�ͣ��ͻ���ּ���鴤���ɴ��Ʋ�����ӿ���_________(���ߡ����͡�)������ϸ�����˷��ԡ�
�����ѡ��1���\��ʵ����
����������һ��ֲ����ǣ�Ӧ��ø�ⷨ���併��ɵõ��������ǵȶ�����Ҫ���ǡ��о���Ա����ɸѡ��һ���ܺϳɰ�����������ø�ľ���ZHB���ش��������⣺
��1�����Ӹ������з������ZHB����ʹ����______________ΪΨһ̼Դ�����������ӹ����Ͽ�������������_________��ѡ��/������������
��2��������ɸѡ�õ���ZHB�������״ͻ�𣬾��¹۲췢�֣���ϸ�������ԡ���˿��ɫ���ܡ��������ɫ���ӡ����������������Ʋ�ZHB����____________��ϸ��/�������������һ�Ʋ⣬�����þ����������pHӦ����________�������¶�ӦΪ_________��
��3����ZHB�������Ƴ���Һ��Ӧ��_____________�����֣��������õ�ZHB�ĵ��������ں����о��������������У���ô���������Ĺؼ���___________________________��
��4�����ⶨZHB�����İ�����������ø�Ļ�����Ӧѡ���±���_________����Һ����ѡ����������Һ��ԭ����________________________________��
����Һ | �������� | ��������ø | NaNO3 | K2HPO4 | MgSO4��7H2O | KCl | FeSO4��7H2O |
�� | 25g/L | - | - | 1g/L | 1g/L | 0.5g/L | 0.01g/L |
�� | 25g/L | 1?g/L | 3g/L | 1g/L | 1g/L | 0.5g/L | 0.01g/L |
�� | 25g/L | - | 3g/L | 1g/L | 1g/L | 0.5g/L | 0.01g/L |


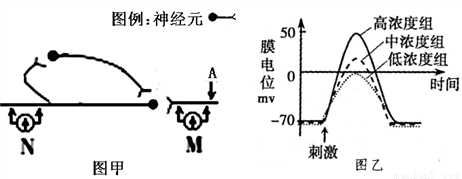
 ��Ũ���Ȼ�����Һ�У������㹻�̼������Ĥ��λ�仯����½�������ԭ����:_________________________________________��
��Ũ���Ȼ�����Һ�У������㹻�̼������Ĥ��λ�仯����½�������ԭ����:_________________________________________��