��Ŀ����
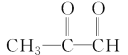
����Ŀ������A����ͼ��ʾ��ת����ϵ(���ֲ�������ȥ)����֪H��ʹ���CCl4��Һ��ɫ��

�ش��������⣺
��1��A�Ľṹ��ʽΪ________��
��2��1 mol E������NaOH��Һ��Ӧʱ������NaOH�����ʵ���Ϊ________ mol��
��3��M��ijЩͬ���칹���ܷ���������Ӧ��д�����е����ֽṹ��ʽ��________________��________________��
��4��д����ѧ����ʽH�D��I��____________________________________________________��
��Ӧ����Ϊ____________________________________________________��
C��H�D��F��____________________________________________________��
��5��Eͨ�����ɱ�ϩ��NaOH��Һ��H2��O2��Br2��Ϊԭ�Ϻϳɣ��밴��A�D��B�D��C�D����������ʽд����Ӧ���̣��������D������ע����Ӧ���͡�____________
���𰸡�CH3CHClCOOCH31OHCCH2COOHOHCCH(OH)CHO��OHCCOOCH3(��ѡ���ּ��ɣ����������𰸾���)nCH2===CHCOOH![]()
 �Ӿ۷�ӦCH3OH��CH2===CHCOOH
�Ӿ۷�ӦCH3OH��CH2===CHCOOH![]() CH2===CHCOOCH3��H2OCH3CH===CH2
CH2===CHCOOCH3��H2OCH3CH===CH2![]()

![]()

![]()
![]()

![]()

��������
��1����ͼʾ��֪��A����4��̼ԭ�ӣ���B��ֻ��3��̼ԭ�ӣ��ɲµ�A��NaOH��Һ�з�Ӧ�õ�B����A��Ӧ���������Ǽ״�����������ΪM���ܷ���������Ӧ����A����ԭ�Ӳ�������̼ԭ���ϣ���A�Ľṹ��ʽΪCH3CHClCOOCH3����2��EΪCH3CH(OH)COOH��ֻ������1 mol NaOH���ʴ�Ϊ��1����3�����ܷ���������Ӧ���ʷ����к���ȩ�������ܵĽṹ�У�OHCCH2COOH��OHCCH(OH)CHO��OHCCOOCH3��OHCCH2OOCH��OHCOCOCH3�ȣ��ʴ�Ϊ��OHCCH2COOH��OHCCH(OH)CHO��OHCCOOCH3(��ѡ���ּ��ɣ����������𰸾���)����4��H��IΪ�Ӿ۷�Ӧ������ʽΪ��nCH2===CHCOOH![]()
![]() ��C��H��FΪ������Ӧ������ʽΪ��CH3OH��CH2===CHCOOH
��C��H��FΪ������Ӧ������ʽΪ��CH3OH��CH2===CHCOOH![]() CH2===CHCOOCH3��H2O����5��E�ĺϳ�·�ߣ�CH3CH===CH2
CH2===CHCOOCH3��H2O����5��E�ĺϳ�·�ߣ�CH3CH===CH2![]()
![]()
![]()
![]()
![]()
![]()
![]()

![]()
![]() ��
��