��Ŀ����
����Ŀ����ͼΪij�ȵ��ط��Ӽ���ֲ��Ŵ�ʾ��ͼ(���еģ�S��S������2����SH��ȥ2��H�γɵ�)����֪���ȵ�����51�������������ɡ���ش��������⣺
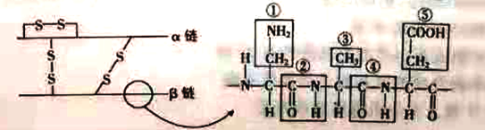
(1)����ȵ��صİ�����Ľṹͨʽ��________________��
(2)����һ���ȵ��ط�����ȫˮ��ɰ����ᣬ����Ҫ����________��ˮ���ӡ�ͼ�зŴ�������ľֲ��ṹ���ܣ���________�ְ����ᣬͼ�������ļ�����________ (������)��
(3)��ͼ��֪��һ���ȵ��ط��������ٺ���________������İ�����________��������Ȼ���
(4)������ȵ��صİ������ƽ����Է�������Ϊ120�����ȵ��ص���Է�������Ϊ______��
���𰸡� 49 3 �ڢ� 3 3 5232
49 3 �ڢ� 3 3 5232
��������
1�����ɵ����ʵĻ�����λ�ǰ����ᣬ��ṹͨʽ��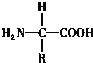 ����ÿ�ְ�����������ٶ�����һ��������һ���Ȼ����Ҷ���һ��������һ���Ȼ�������ͬһ��̼ԭ���ϣ����̼ԭ�ӻ�����һ�����һ��R����������IJ�ͬ����R���IJ�ͬ��
����ÿ�ְ�����������ٶ�����һ��������һ���Ȼ����Ҷ���һ��������һ���Ȼ�������ͬһ��̼ԭ���ϣ����̼ԭ�ӻ�����һ�����һ��R����������IJ�ͬ����R���IJ�ͬ��
2���������ں�������ͨ����ˮ�����γɶ�����������ˮ������ָһ����������ӵ��Ȼ���-COOH ������һ����������ӵİ�����-NH2�������ӣ�ͬʱ�ѳ�һ����ˮ�Ĺ��̣���������������Ļ�ѧ�����ļ�����ṹʽ��-CO-NH-���������γɶ��Ĺ����е���ؼ��㣺�ļ���=��ȥˮ������=��������һ�����������백�����Ȼ���=������+R���к��еİ������Ȼ��������ٺ��е����백�����Ȼ���=����������ԭ����=�ļ���+������+R���ϵĵ�ԭ����=���������е�ԭ����������ԭ����=�ļ���+2��������+R���ϵ���ԭ����=������������ԭ������һ��ȥˮ�������������ʵ���Է�������=��������Ŀ��������ƽ����Է�������һ��ȥˮ��������18��
2�����������ʶͼ������֪���ȵ��ط�����51�������������ɣ���2���������ɣ�����3������������ȵ����γɹ�������ȥˮ����49�����γ��ļ�49��������ͼ�зŴ�������ľֲ��ṹ���ܿ�֪��ͼ�Тڢ�Ϊ�ļ����٢ۢ�Ϊ�������R�������ڢ٢ۢݸ�����ͬ���ʸþֲ������ְ�������ˮ���϶��ɡ�����ͼ�Т٢ۢݿ�֪���ȵ�������������İ������Ȼ�����3����
��1��������Ľṹͨʽ��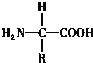 ����ÿ�ְ�����������ٶ�����һ��������һ���Ȼ����Ҷ���һ��������һ���Ȼ�������ͬһ��̼ԭ���ϣ����̼ԭ�ӻ�����һ�����һ��R����������IJ�ͬ����R���IJ�ͬ��
����ÿ�ְ�����������ٶ�����һ��������һ���Ȼ����Ҷ���һ��������һ���Ȼ�������ͬһ��̼ԭ���ϣ����̼ԭ�ӻ�����һ�����һ��R����������IJ�ͬ����R���IJ�ͬ��
��2��һ���ȵ��ط����γɹ������ѵ���49���ӵ�ˮ����һ���ȵ��ط�����ȫˮ��ɰ����ᣬ����Ҫ����49���ӵ�ˮ���������Ϸ�����֪��ͼ�зŴ�������ľֲ��ṹ���ܣ�����R���IJ�ͬ����3�ְ����ᣬͼ�Тڢ������ļ���
��3����ͼ��֪��һ���ȵ��ط�������2���������Ҿֲ��Ŵֵ�R������һ������İ�����һ��������Ȼ���������ٺ���3������İ�����3��������Ȼ���
��4��������ȵ��صİ������ƽ����Է�������Ϊ120���ȵ��ط�����51�������������ɣ��γɹ������ѵ���49���ӵ�ˮ���γ���3������������ȵ��ص���Է�������Ϊ��120��51-49��18-3��2=5232��

����Ŀ��Ϊ���������ض����ܵĦ�-����ø���о���Ա��ij�ֺ���ϸ���п�¡�˦�-����ø����1656������ԣ������û��̴����Ʊ���-����ø��ʵ�����̼���ͼ����ش��������⣺
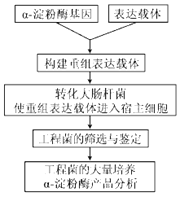
��1����ȡ��-����ø���������п�¡�����õļ�����_________��
��2��Ϊ�˱���������DNAƬ��������������ӣ����������_______�ˣ���5����3��������������ø��λ�㣬�ҳ���������������Ƽ��벻ͬ��������ø��λ�㣬��ҪĿ����_______________
��3����������ʱ����Ӧ���¶Ⱥ�ʱ������ݾ�����������趨������ѡ����________���趨�������йأ�________���趨������Ƭ�εij����йء�������ţ�
�ٱ����¶� ���˻��¶� �������¶� �ܱ���ʱ�� ���˻�ʱ�� ������ʱ��
��4����ͼ��ʾɸѡ��õĹ��̾��б����-����ø��mRNA�IJ��ּ�����У�
![]()
ͼ�����߿���mRNAƬ�ΰ���______�������ӣ������߿�������δ֪��Ԥ�����߿��ĵ�һ�������������_______ �֡�
��5����ù��̾�����Ħ�-����ø��Ϊ̽��Ӱ��ø���Ե����أ���Ũ��Ϊ1%�Ŀ����Ե���Ϊ����ⶨø���ԣ�������£�
����Һ | 50 mmol/L Na2HPO4-KH2PO4 | 50 mmol/L Tris-HCl | 50 mmol/L Gly-NaOH | ||||||||||
pH | 6.0 | 6.5 | 7.0 | 7.5 | 7.5 | 8.0 | 8.5 | 9.0 | 9.0 | 9.5 | 10.0 | 10.5 | |
ø��Ի���% | 25.4 | 40.2 | 49.8 | 63.2 | 70.1 | 95.5 | 99.5 | 85.3 | 68.1 | 63.7 | 41.5 | 20.8 | |
��������ʵ�����������жϸæ�-����ø������ߵ�����Ϊ_________________________ ��