��Ŀ����
����Ŀ����ͼΪij�������õ�ˮ����̬ϵͳ������������λkJ/��m2a-1��ʾ��ͼ����ͼ��Ϣ���ش��������⣺
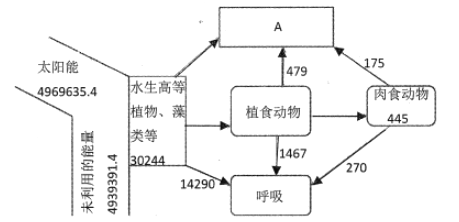
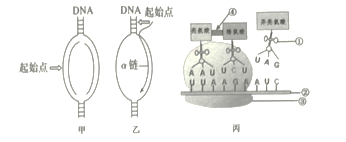
��1������̬ϵͳ�ɷַ�����A��ʾ______������Ҫ�ֲ��ڸ�ˮ��Ⱥ���______�㡣
��2���ɲ���________������ij��ʳ�������Ⱥ������
��3��ֲʳ���ﵽ��ʳ�������������Ч��______%������lλС���� ����������������______��ʽ�ͷŵ���Χ�ռ䣻ͼ�����ݱ��ֲ��䣬��Ϊ��ʳ�������Ӳ����˹����ϣ���______��Ӱ��/��Ӱ�죩ֲʳ��������ʳ�������������Ч�ʡ�
���𰸡��ֽ��� �� ��־�ز� 18.6 ���� ��Ӱ��
��������
��̬ϵͳ�Ľṹ��ָ��̬ϵͳ����ɳɷֺ�Ӫ���ṹ����ɳɷְ�������������ʺ������������ߡ������ߺͷֽ��ߣ�Ӫ���ṹ��ָʳ������ʳ��������̬ϵͳҲ������ָ������������Ⱥ�䡣����������̬ϵͳ�Ļ�ʯ������������̬ϵͳ����Ҫ��ɳɷ֣��ֽ�������̬ϵͳ����ȱ�ٵijɷ֡�
��1������̬ϵͳ�ɷַ�����A��ʾ�ֽ��ߣ��ֽ�����Ҫ�ֲ��ڸ�ˮ��Ⱥ��ĵײ㡣
��2����ʳ����һ��������ǿ�����Χ�Ϲ㣬���Ե�������Ⱥ������Ҫ���ñ�־�ز�����
��3����������Ӫ����֮�����������Ч�ʱ�ʾ������ͬ�����ı�ֵ������ֲʳ���ﵽ��ʳ�������������Ч��Ϊ445�£�479+1467+445����100%=18.6%����������������������ʽ�ͷŵ���Χ�ռ䣻ͼ�����ݱ��ֲ��䣬��Ϊ��ʳ�������Ӳ����˹����ϣ���ʳ�����ֲʳ�����õ��������䣬��Ӱ��ֲʳ��������ʳ�������������Ч�ʡ�

����Ŀ������������ȤС��ֱ�Խ�ĸ��ϸ��������ʽ���������µ�̽��ʵ�顣������������
��1������ȤС����̽���ľ��������ǣ���ĸ���Ƿ������������������¾��ܲ���CO2�����ṩ�����ף�ÿ����������ʵ��װ����ͼ��a��d����ʾ��
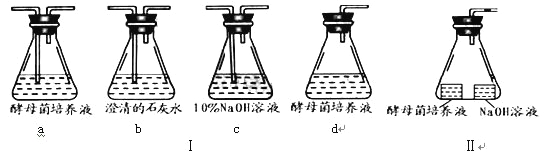
�� �����ʵ��Ŀ��ѡ��װ����ţ�������ʵ�����װҪ������װ�ÿ��ظ�ʹ�ã������������µ�װ����ţ�_________________�����������µ�װ����ţ�_________________��
�� װ����cƿ�������ǣ�_________________________________________________��
��2������ȤС������ͼ����ʾװ�ã���Ƥ���ϵ����Ϊ���к�ɫҺ�εĿ̶Ȳ����ܣ���̽����ĸ����ϸ���������͡�
�� ��õ�ʵ����ۻ�����ͬʱ���ö���ʵ�飨ʵ������л������ص�Ӱ����Բ��ƣ�������ʵ��װ�ã������װ�ñ��Ϊ��Ϊ______________________________________________��
�� ��Ԥ�����������ϵ�������д�±���
��� | װ���к�ɫҺ�ε��ƶ����� | ���� | |
װ�â� | װ�â� | ||
1 | ��____________ | �� ���ƶ� | ֻ������������ |
2 | �� ���ƶ� | ��____________ | ֻ������������ |
3 | �� ������ | ��____________ | �Ƚ��������������ֽ����������� |
�� ����ĸ�����ĵ�O2Ϊ3mol�����ͷŵ�CO2Ϊ9mol�����ĸ�������������������ǵ���������������________����