��Ŀ����
����Ŀ����ͼ������ڻ�����̬���ڻ����йأ����ͼ�ش��������⣺
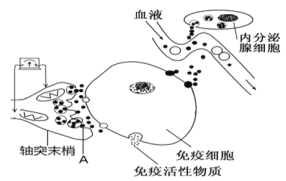
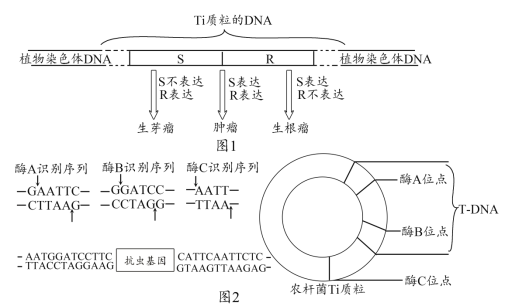
��1����ͼ��֪��������ϸ�����������ٵĵ��ڣ��ȿ�ͨ��ͻ��ǰĤ�ͷ�______ֱ�ӽ��У�����ͨ���й�______���У����߾�������ϸ����ϸ��Ĥ������Ӧ�����ϣ�������ϸ��Ĥ����_________________���ܡ�
��2��ͼ����Ԫδ�ܴ̼�ʱ�͵����Ƶ�ָ����____ƫת�� A�����ź�ת������Ϊ_______________��
��3�������ڻ���ά����̬�ĵ��ڻ���Ϊ_________________��
���𰸡����� ���� ����ϸ�������Ϣ���� �� ���źš���ѧ�ź� ��--��Һ--���ߵ�������
��������
��ͼ��ͻĩ���е�ͻ��С���ͷ�A���ʣ�����������ϸ�������壻�ڷ����ٷ��ڼ��أ�ͨ��ѪҺ���䣬����������ϸ���ϵ����壬˵������ϸ���ܵ��ͼ��ص��ڡ�
(1)������ͼ����֪��������ϸ���������ʾ������ϸ�������ڣ�ͻ��ǰĤ���ͷ����ʣ�������ͻ����Ĥ������ϸ���ϵ����壬��������ϸ���������������ʣ�����ϸ���Ҳ���ʾ������ϸ�����ڷ����ٷ��ڵļ��صĵ��ڣ��ڷ����ٷ��ڵļ��ؾ�����Һ���䵽��ϸ�������ϸ��Ĥ�ϵ������ϣ�������ϸ���������������ʽ��е��ڣ���Ϣ�������ϸ��Ĥ�ϵ������Ͻ������ڰ�ϸ�����������������ϸ��Ĥ������Ϣ�����Ĺ��ܡ�
(2)��Ϊ��ϢʱĤ���λ������λ,Ĥ�ڵ�λ�Ǹ���λ����Ϊ������ָ��ƫת������������ƶ��ķ���������λ����λ,ͼ�е�ָ�������ƫת��ͼ��A�����ͷ����ʣ��źŵ�ת�������ǵ��ź�ת��Ϊ��ѧ�źš�
(3)����ͼ����֪��,������̬���ڵ������ڡ����ߵ��ں���Һ���ڣ���ͼʾ�������ֻ���ά����̬����Ҫ���ڻ���Ϊ��--��Һ--���ߵ������硣

����Ŀ�����й��Ŵ����д�ͳ���ͼ����Ĺ㷺Ӧ�ñ�����ơ����������Լ����顢�ݲ˵������ȣ��������\���ķ�չ�����������ִ����\����������֭����øϴ�·ۺ���ȡ���ܲ��غ�������Դ�ƾ��ȣ�ͬʱ����ֲ����֯����������������ֳ������ľ�����벻ͬ������������������ʵ�ʡ���������ѧ����ѧ֪ʶ������ͬ���\����ʵ���е�Ӧ�á�
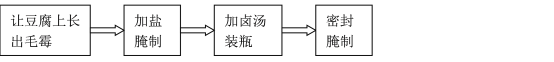
��1���������ҹ��Ŵ��Ͷ����������һ�־�������͵Ĵ�ʳƷ�������Ǹ�������������ʾ��ͼ��
���ִ���ѧ�о�������������������˶����ķ��ͣ���������Ҫ���õ���________________��
�ڸ���������ԭ����______________________________________________________��
��2���ݲ��������ճ������бȽ�ϲ����һ��ʳƷ�������ݲ���ȴ�����������Ρ�������������������������ﵽ0.3g��0.5gʱ���������ж����ﵽ3gʱ��������������
�������ݲ˵�ԭ����_________________________________________________________��
�ڲ���ָ�꼰����������������ijЩ��ѧ���ʷ�����Ӧ���γ�_____________ɫȾ�ϡ���ʹ�ݲ���Ʒ��һϵ����֪Ũ�ȵ�����������Һ�ֱ��뻯ѧ���ʷ�����ɫ��Ӧ��Ȼ��ͨ��___________�����Թ�����ݲ�Һ�����������εĺ�����
KH2PO4 | 1.4g |
Na2HPO4 | 2.1g |
MgSO4.7H2O | 0.2g |
������ | 10g |
���� | 1g |
��֬ | 15g |
�����������ܽ��,������ˮ���ݵ�1000ml | |
��3��������һ����Ҫ��ũҵ���ϣ���������ϸ���ķֽ⣬�Ͳ��ܸ��õر�ֲ�����á������������е�����������������࣬���������������������������з�����ֽ����ص�ϸ����Ŀ�ľ������ش����⣺
�ٴ��������ܷ�����ֲ����֯������_______��������_____________________��
����������������_________��������
�ۡ�Ŀ�ľ�����������ĵ�Դ��̼Դ�ֱ������������е�___________��___________��