��Ŀ����
����Ŀ��A��B��C��D��EΪԪ�����ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������A��B��C��D��E��˳������A�������������Ǵ�����������2����C��E������������ȣ�E������������к���60%��D��C���γ�D2C��D2C2�������ӻ������д���пհף�
��1��д����������Ԫ�ص�Ԫ�ط��ţ�A___��B___��C___��D____��E___��
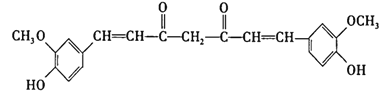
��2��д��D2C2�ĵ���ʽ_________��AC2�Ľṹʽ___________��
��3���õ���ʽ��ʾ AC2��������γɹ���__________________��
���𰸡�C N O Na S ![]() O=C=O
O=C=O ![]()
��������
A��B��C��D��EΪԪ�����ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������A��B��C��D��E��˳������A�������������Ǵ�����������2������AΪC��C��E������������ȣ�E������������к���60%��������ΪSO3����EΪS��CΪO��D��C���γ�D2C��D2C2�������ӻ������DΪNa���Դ������
(1)A�������������Ǵ�����������2������AΪC��C��E�������������,E������������к���60%��������ΪSO3����EΪS��CΪO��D��C���γ�D2C��D2C2�������ӻ������DΪNa��A. B. C. D.EΪԪ�����ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������A. B. C. D.E��˳��������BΪN��
�ʴ�Ϊ��C��N��O��Na��S��
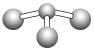
(2)D2C2�γɵ��ǹ������ƣ������ʽΪ![]() ��AC2ΪCO2�������ں�̼������˫�����ṹʽΪO=C=O��
��AC2ΪCO2�������ں�̼������˫�����ṹʽΪO=C=O��
�ʴ�Ϊ��![]() ��O=C=O��
��O=C=O��
(3)�õ���ʽ��ʾCO2��������γɹ���Ϊ![]() ��
��
�ʴ�Ϊ��![]() ��
��

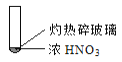
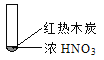
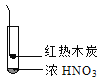
����Ŀ������ʵ���о��к���ɫ����������Աȷ������ý��۴������
|
|
|
�� | �� | �� |
A. �ɢ��еĺ���ɫ���壬�ƶϲ���������һ���ǻ������
B. ���к���ɫ���岻�ܱ���ľ̿��Ũ���ᷢ���˷�Ӧ
C. �ɢ�˵��Ũ������лӷ��ԣ����ɵĺ���ɫ����Ϊ��ԭ����
D. �۵���������м���CO2���ɴ�˵��ľ̿һ����Ũ���ᷢ���˷�Ӧ