��Ŀ����
����25 ��ʱ0.1 mol��L��1�İ�ˮ����ش��������⣺
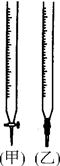
��1������ˮ�м�����������粒��壬һˮ�ϰ��ĵ���ƽ��________(����������ҡ�����)�ƶ�����ʱ��Һ��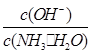 ________(���������С�����䡱)��
________(���������С�����䡱)��
��2������ˮ�м����Ũ��ϡ���ᣬʹ��ǡ���кͣ�д����Ӧ�����ӷ���ʽ��_________________��������Һ��pH________7(���������<������)��
��3������ˮ�м���ϡ��������Һ��pH��7����ʱ[NH4+]��a mol��L��1����c(SO42-)��________��
��4������ˮ�м���pH��1�����ᣬ�Ұ�ˮ������������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����________________________________________��
��1������ˮ�м�����������粒��壬һˮ�ϰ��ĵ���ƽ��________(����������ҡ�����)�ƶ�����ʱ��Һ��
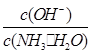 ________(���������С�����䡱)��
________(���������С�����䡱)����2������ˮ�м����Ũ��ϡ���ᣬʹ��ǡ���кͣ�д����Ӧ�����ӷ���ʽ��_________________��������Һ��pH________7(���������<������)��
��3������ˮ�м���ϡ��������Һ��pH��7����ʱ[NH4+]��a mol��L��1����c(SO42-)��________��
��4������ˮ�м���pH��1�����ᣬ�Ұ�ˮ������������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����________________________________________��
��1������ ��С ��2��NH3��H2O��CH3COOH��CH3COO����NH4����H2O �� ��3��
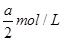
��4��c(NH4��)��c(SO42��)��c(H��)��c(OH��)

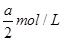
��4��c(NH4��)��c(SO42��)��c(H��)��c(OH��)
�����������1����ˮ�м�����������粒��壬����淋��������笠����ӻ�����һˮ�ϰ��ĵ��룬ƽ�������ƶ�����ʱ��Һ��
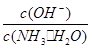 ��С ��2��NH3��H2O��CH3COOH��CH3COO����NH4����H2O ����Ϊǡ���кͣ���Һ�����ԣ�������Һ��pH��7 ��3�����ݵ���غ��У�c(NH4��)��c(H��)��2c(SO42��)��c(OH��)������Ϊ��Һ��pH��7������У�c(SO42��)��
��С ��2��NH3��H2O��CH3COOH��CH3COO����NH4����H2O ����Ϊǡ���кͣ���Һ�����ԣ�������Һ��pH��7 ��3�����ݵ���غ��У�c(NH4��)��c(H��)��2c(SO42��)��c(OH��)������Ϊ��Һ��pH��7������У�c(SO42��)�� c(NH4��)��
c(NH4��)��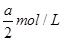 ����4������ˮ�м���pH��1�����ᣬ�Ұ�ˮ������������Ϊ1��1����õ���������淋���Һ����Ϊ�������ӵ�ˮ�⣬ʹ����Һ�����ԣ���������¹�ϵ��c(NH4��)��c(SO42��)��c(H��)��c(OH��)
����4������ˮ�м���pH��1�����ᣬ�Ұ�ˮ������������Ϊ1��1����õ���������淋���Һ����Ϊ�������ӵ�ˮ�⣬ʹ����Һ�����ԣ���������¹�ϵ��c(NH4��)��c(SO42��)��c(H��)��c(OH��)
��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ